Potassium hexacyanidoferrate(II) is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when used in pigments.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.

Iodic acid is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
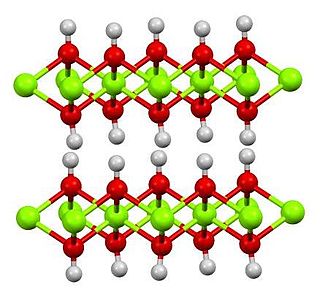
Iron (II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron (II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".

Iron(III) phosphate, also ferric phosphate, is the inorganic compound with the formula FePO4. Four polymorphs of anhydrous FePO4 are known. Additionally two polymorphs of the dihydrate FePO4·(H2O)2 are known. These materials have attracted much interest as potential cathode materials in batteries.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure.

Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is a lime-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.

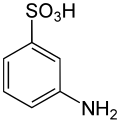
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.
Boron triiodide is a chemical compound of boron and iodine with chemical formula BI3. It has a trigonal planar molecular geometry.
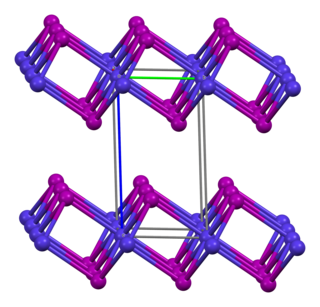
Cobalt(II) iodide or cobaltous iodide are the inorganic compounds with the formula CoI2 and the hexahydrate CoI2(H2O)6. These salts are the principal iodides of cobalt.

Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH)
2, consisting of divalent cobalt cations Co2+
and hydroxide anions OH−
. The pure compound, often called the "beta form" is a pink solid insoluble in water.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.

In materials science, a disappearing polymorph is a form of a crystal structure that is suddenly unable to be produced, instead transforming into a different crystal structure with the same chemical composition during nucleation. Sometimes the resulting transformation is extremely hard or impractical to reverse, because the new polymorph may be more stable. It is hypothesized that contact with a single microscopic seed crystal of the new polymorph can be enough to start a chain reaction causing the transformation of a much larger mass of material. Widespread contamination with such microscopic seed crystals may lead to the impression that the original polymorph has "disappeared". In a few cases such as progesterone and paroxetine hydrochloride, the disappearance is global, and it is suspected that it is because earth's atmosphere is permeated with tiny seed crystals. It is believed that seeds as small as a few million molecules is sufficient for converting one morph to another, making unwanted disappearance of morphs particularly difficult to prevent.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.