| Names | |
|---|---|
| Preferred IUPAC name Methanedithiol | |
| Other names Dimercaptomethane | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.166.842 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CH4S2 | |
| Molar mass | 80.16 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 58 °C (136 °F; 331 K) |
Refractive index (nD) | 1.581 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
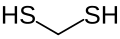
Methanedithiol is an organosulfur compound with the formula H2C(SH)2. A seldom used chemical, it forms when formaldehyde reacts with hydrogen sulfide under pressure:
- CH2O + 2 H2S → H2C(SH)2 + H2O
This reaction competes with formation of trithiane:
- 3 H2C(SH)2 → [H2CS]3 + 3 H2S
Methanedithiol forms a solid dibenzoate derivative upon treatment with benzoic anhydride: [1]
- 3 H2C(SH)2 + 2 (C6H5CO)2O → H2C[SC(O)C6H5]2 + 2 C6H5CO2H
Methanetrithiol is also known.