 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Methyl phenylacetate | |
| Other names Methyl 2-phenylacetate Methyl benzene acetate | |
| Identifiers | |
3D model (JSmol) | |
| 878795 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.674 |
| EC Number |
|
| MeSH | C024906 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.1745 g mol−1 |
| Appearance | Colorless liquid |
| Density | 1.055±0.060 g/cm3 |
| Melting point | 50 °C (122 °F; 323 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| 2070 mg/L | |
| Vapor pressure | 17.3 Pa |
| −92.73×10−6 cm3/mol | |
Refractive index (nD) | 1.505±0.020 at 20 °C |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 90.6 °C (195.1 °F; 363.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
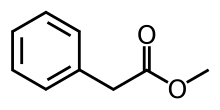
Methyl phenylacetate is an organic compound that is the methyl ester of phenylacetic acid, with the structural formula C6H5CH2CO2CH3. It is a colorless liquid that is only slightly soluble in water, but soluble in most organic solvents.
Methyl phenylacetate has a strong odor similar to honey. This compound also occurs in brandy, capsicum, coffee, honey, pepper, and some wine. It is used in the flavor industry and in perfumes to impart honey scents. [1]
Methyl phenyldiazoacetate, precursor to cyclopropanation agents, is prepared by treating methyl phenylacetate with p-acetamidobenzenesulfonyl azide in the presence of base. [2]
