
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Phosphorus trichloride is a inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts violently with water to release hydrogen chloride.
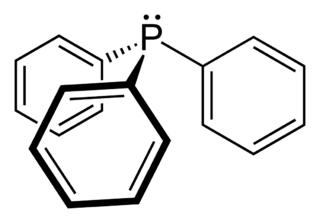
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
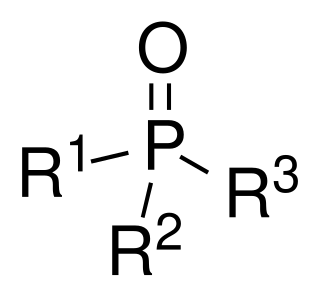
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3).

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.
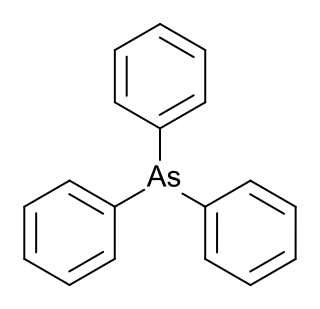
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.

Trimethylphosphine is the organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common ligand in coordination chemistry.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.

Triphenyl phosphite is the organophosphorus compound with the formula P(OC6H5)3. It is a colourless viscous liquid.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.
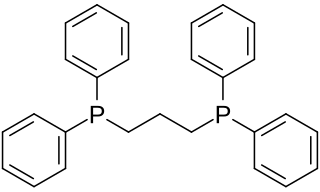
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.

Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. It is colorless air sensitive liquid. It is a member of series (CH3)3-n(C6H5)2P that also includes n = 0, n = 2, and n = 3 that are often employed as ligands in metal phosphine complexes.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. Covalently bonded metal halides may be discrete molecules, such as uranium hexafluoride, or they may form polymeric structures, such as palladium chloride.

trans-Bis(dinitrogen)bis[1,2-bis(diphenylphosphino)ethane]molybdenum(0) is a coordination complex with the formula Mo(N2)2(dppe)2. It is a relatively air stable yellow-orange solid. It is notable as being the first discovered dinitrogen containing complex of molybdenum.
In organophosphorus chemistry, an aminophosphine is a compound with the formula R3−nP(NR2)n where R = H or an organic substituent, and n = 0, 1, 2. At one extreme, the parent H2PNH2 is lightly studied and fragile, but at the other extreme tris(dimethylamino)phosphine (P(NMe2)3) is commonly available. Intermediate members are known, such as Ph2PN(H)Ph. These compounds are typically colorless and reactive toward oxygen. They have pyramidal geometry at phosphorus.
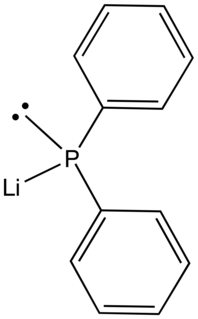
Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula (C6H5)2PLi. It is an air-sensitive solid that is used in the preparation of diphenylphosphino compounds. As an ether complex, the lithium salt is dark red.
Triethylphosphine is the organophosphorus compound with the formula P(CH2CH3)3, commonly abbreviated as PEt3. It is a colorless liquid with an unpleasant odor characteristic of alkylphosphines. The compound is a common ligand in organometallic chemistry, such as in auranofin.
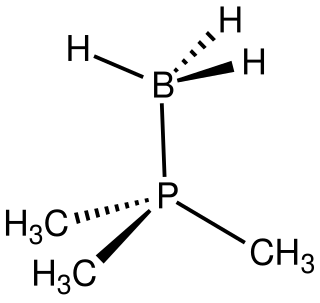
In chemistry, phosphine-boranes are organophosphorus compounds with the formula R3-nHnPBH3. They are Lewis acid-Lewis base adducts derived from organophosphines (PR3-nHn) and borane (BH3). They are generally colorless or white solids. Since these adducts are air-stable, they represent a protected form of the parent organophosphine.
















