Related Research Articles

A hemeprotein, or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both.
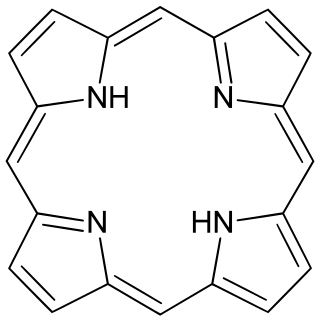
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (porphyra), meaning purple.

In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

A corrole is an aromatic tetrapyrrole. The corrin ring is also present in cobalamin (vitamin B12). The ring consists of nineteen carbon atoms, with four nitrogen atoms in the core of the molecule. In this sense, corrole is very similar to porphyrin.

A chromophore is the part of a molecule responsible for its color. The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Harry Barkus Gray is the Arnold O. Beckman Professor of Chemistry at California Institute of Technology.

Daniel George Nocera is an American chemist, currently the Patterson Rockwood Professor of Energy in the Department of Chemistry and Chemical Biology at Harvard University. He is a member of the National Academy of Sciences and the American Academy of Arts and Sciences. In 2006 he was described as a "major force in the field of inorganic photochemistry and photophysics". Time magazine included him in its 2009 list of the 100 most influential people.
Jeremy Keith Morris Sanders is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015.

Didier Astruc carried out his studies in chemistry in Rennes. After a Ph. D. with professor R. Dabard in organometallic chemistry, he did post-doctoral studies with professor R. R. Schrock at the Massachusetts Institute of Technology Cambridge, Massachusetts, in the U.S. and later a sabbatical year with professor K. P. C. Vollhardt at the University of California at Berkeley. He became a CNRS Director of research in Rennes, then in 1983 full Professor of Chemistry at the University Bordeaux 1. He is known for his work on “Electron-Reservoir” complexes and dendritic molecular batteries, catalytic processes using nanoreactors and molecular recognition using gold nanoparticles and metallodendrimers. He is the author of three books, scientific publications and the editor of five books or special issues. He has been a member of the National CNRS committee from 2000 to 2008 and the President of the Coordination Chemistry Division of the Société Française de Chimie from 2000 to 2004. Didier Astruc is on the Thompson-Reuters list of the top 100 chemists who have achieved the highest citation impact scores for their chemistry papers published between 2000 and 2010. and on the list of the Highest Cited Researchers 2015 and 2016 (Thomson-Reuters). and 2017

Eluvathingal Devassy Jemmis or E. D. Jemmis is a professor of theoretical chemistry at the Indian Institute of Science, Bangalore, India. He was the founding director of Indian Institute of Science Education and Research, Thiruvananthapuram (IISER-TVM). His primary area of research is applied theoretical chemistry with emphasis on structure, bonding and reactivity, across the periodic table of the elements. Apart from many of his contributions to applied theoretical chemistry, an equivalent of the structural chemistry of carbon, as exemplified by the Huckel 4n+2 Rule, benzenoid aromatics and graphite, and tetrahedral carbon and diamond, is brought in the structural chemistry of boron by the Jemmis mno rules which relates polyhedral and macropolyhedral boranes to allotropes of boron and boron-rich solids. He has been awarded Padma Shri in Science and Engineering category by the Government of India.
Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.
Gregory S. Girolami is the William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois at Urbana-Champaign. His research focuses on the synthesis, properties, and reactivity of new inorganic, organometallic, and solid state species. Girolami has been elected a fellow of the American Association for the Advancement of Science, the Royal Society of Chemistry, and the American Chemical Society.
Nenad Trinajstić was a Croatian chemist and one of pioneers of the chemical graph theory.

Carbene radicals are a special class of organometallic carbenes. The carbene radical can be formed by one-electron reduction of Fischer-type carbenes using an external reducing agent, or directly upon carbene formation at an open-shell transition metal complex using diazo compounds and related carbene precursors. Cobalt(III)-carbene radicals have found catalytic applications in cyclopropanation reactions, as well as in a variety of other catalytic radical-type ring-closing reactions.
Light harvesting materials harvest solar energy that can then be converted into chemical energy through photochemical processes. Synthetic light harvesting materials are inspired by photosynthetic biological systems such as light harvesting complexes and pigments that are present in plants and some photosynthetic bacteria. The dynamic and efficient antenna complexes that are present in photosynthetic organisms has inspired the design of synthetic light harvesting materials that mimic light harvesting machinery in biological systems. Examples of synthetic light harvesting materials are dendrimers, porphyrin arrays and assemblies, organic gels, biosynthetic and synthetic peptides, organic-inorganic hybrid materials, and semiconductor materials. Synthetic and biosynthetic light harvesting materials have applications in photovoltaics, photocatalysis, and photopolymerization.
David Nathan Beratan is an American chemist and physicist, the R.J. Reynolds Professor of Chemistry at Duke University. He has secondary appointments in the departments of Physics and Biochemistry. He is the Director of the Center for Synthesizing Quantum Coherence, a NSF Phase I Center for Chemical Innovation.

Stephen L. Craig is the William T. Miller Professor of Chemistry at Duke University. He is the director of the Center for Molecularly Optimized Networks, a NSF Center for Chemical Innovation.

Atsuhiro Osuka is a research professor of organic chemistry in the Department of Chemistry, Graduate School of Science, Kyoto University (Japan). He is recognized in the fields of porphyrinoid chemistry for his works in extended π-electron systems and its tunable aromatic behaviors.

Phosphorus-centered porphyrins are conjugated polycyclic ring systems consisting of either four pyrroles with inward-facing nitrogens and a phosphorus atom at their core or porphyrins with one of the four pyrroles substituted for a phosphole. Unmodified porphyrins are composed of pyrroles and linked by unsaturated hydrocarbon bridges often acting as multidentate ligands centered around a transition metal like Cu II, Zn II, Co II, Fe III. Being highly conjugated molecules with many accessible energy levels, porphyrins are used in biological systems to perform light-energy conversion and modified synthetically to perform similar functions as a photoswitch or catalytic electron carriers. Phosphorus III and V ions are much smaller than the typical metal centers and bestow distinct photochemical properties unto the porphyrin. Similar compounds with other pnictogen cores or different polycyclic rings coordinated to phosphorus result in other changes to the porphyrin’s chemistry.
References
- 1 2 3 4 "Duke Chemistry". Duke University Chemistry. Retrieved 16 July 2019.
- ↑ "Duke aiM Program". Duke aiM Program. Retrieved 28 January 2022.
- ↑ "Academic Family Tree – William C. Trogler". Academic Tree - Trogler. Retrieved 16 July 2019.
- ↑ "Therien Lab Website".
- ↑ "Google Scholar" . Retrieved 20 August 2020.
- ↑ "John Simon Guggenheim Foundation | 2020 Fellows Press Release". gf.org. Retrieved 2020-04-12.
- ↑ "Duke Center for Biomolecular and Tissue Engineering".
- ↑ "Francqui Foundation".
- ↑ "AAAS Fellows 2005" (PDF).
- ↑ "Recipients of the Philadelphia Section Award".
- ↑ "SPP/JPP Young Investigator Awards".
- ↑ "Past Sloan Fellows". Archived from the original on 2018-03-14. Retrieved 2019-07-17.
- ↑ "NSF Young Investigator: Electron Transfer in Metallo- Porphyrins".
- ↑ "Beckman Young Investigators, Michael J. Therien".
- ↑ "Searle Scholars, Michael J. Therien".