
Lysergic acid diethylamide, commonly known as LSD, is a potent psychedelic drug that intensifies thoughts, emotions, and sensory perception. Often referred to as acid or lucy, LSD can cause mystical, spiritual, or religious experiences. At higher doses, it primarily induces visual and auditory hallucinations. While LSD does not cause physical addiction, it can lead to adverse psychological reactions, such as anxiety, paranoia, and delusions. Additionally, it may trigger "flashbacks," also known as hallucinogen persisting perception disorder (HPPD), where individuals experience persistent visual distortions after use.

Psilocybin, also known as 4-phosphoryloxy-N,N-dimethyltryptamine (4-PO-DMT), and formerly sold under the brand name Indocybin, is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. Psilocybin is itself biologically inactive but is quickly converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to those of other classical psychedelics. Effects include euphoria, hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. It can also cause adverse reactions such as nausea and panic attacks.

Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states and a perceived "expansion of consciousness". Also referred to as classic hallucinogens or serotonergic hallucinogens, the term psychedelic is sometimes used more broadly to include various types of hallucinogens, such as those which are atypical or adjacent to psychedelia like salvia and MDMA, respectively.
Psychedelic therapy refers to the proposed use of psychedelic drugs, such as psilocybin, ayahuasca, LSD, psilocin, mescaline (peyote), DMT, 5-MeO-DMT,Ibogaine,MDMA, to treat mental disorders. As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.
The Multidisciplinary Association for Psychedelic Studies (MAPS) is an American nonprofit organization working to raise awareness and understanding of psychedelic substances. MAPS was founded in 1986 by Rick Doblin and is now based in San Jose, California.

Hallucinogen persisting perception disorder (HPPD) is a non-psychotic disorder in which a person experiences apparent lasting or persistent visual hallucinations or perceptual distortions after using drugs, including but not limited to psychedelics, dissociatives, entactogens, tetrahydrocannabinol (THC), and SSRIs. Despite being designated as a hallucinogen-specific disorder, the specific contributory role of psychedelic drugs is unknown.

Ibogaine is a psychoactive indole alkaloid obtained either by extraction from plants in the family Apocynaceae such as Tabernanthe iboga, Voacanga africana, and Tabernaemontana undulata or by semi-synthesis from the precursor compound voacangine, another plant alkaloid. The total synthesis of ibogaine was described in 1956. Structural elucidation by X-ray crystallography was completed in 1960.
The Beckley Foundation is a UK-based think tank and UN-accredited NGO, dedicated to activating global drug policy reform and initiating scientific research into psychoactive substances. The foundation is a charitable trust which collaborates with leading scientific and political institutions worldwide to design and develop research and global policy initiatives. It also investigates consciousness and its modulation from a multidisciplinary perspective, working in collaboration with scientists. The foundation is based at Beckley Park near Oxford, United Kingdom. It was founded in 1998, and is directed by Amanda Feilding, Countess of Wemyss.

A psychoactive drug, mind-altering drug, or consciousness-altering drug is a chemical substance that changes brain function and results in alterations in perception, mood, consciousness, cognition, or behavior. The term psychotropic drug is often used interchangeably, while some sources present narrower definitions. These substances may be used medically; recreationally; to purposefully improve performance or alter consciousness; as entheogens for ritual, spiritual, or shamanic purposes; or for research, including psychedelic therapy. Physicians and other healthcare practitioners prescribe psychoactive drugs from several categories for therapeutic purposes. These include anesthetics, analgesics, anticonvulsant and antiparkinsonian drugs as well as medications used to treat neuropsychiatric disorders, such as antidepressants, anxiolytics, antipsychotics, and stimulants. Some psychoactive substances may be used in detoxification and rehabilitation programs for persons dependent on or addicted to other psychoactive drugs.
Charles Grob is a professor of Psychiatry & Biobehavioral Sciences and Pediatrics and director of the Division of Child and Adolescent Psychiatry at Harbor–UCLA Medical Center. He received his two BS degrees from Oberlin College and Columbia University, before getting an MD from the State University of New York, Downstate Medical Center. Grob's research interests include anxiety and mood disorders and also self-medication and substance abuse. The FDA approved one of his Phase 1 studies to study the psychological and physiological effects of MDMA and the hallucinogen ayahuasca.

Psychedelic microdosing involves consuming sub-threshold doses (microdoses) of serotonergic psychedelic drugs like LSD and psilocybin to potentially enhance creativity, energy, emotional balance, problem-solving abilities, and to address anxiety, depression, and addiction. This practice has gained popularity in the 21st century. A June 2024 report by the RAND Corporation suggests that among adults in the United States reporting the use of psilocybin in the past year, nearly half reported microdosing the last time they used it.

Psilocybin therapy is the use of psilocybin in treating a range of mental health conditions, such as depression, anxiety, addictions, obsessive compulsive disorder (OCD), and psychosis. It is one of several forms of psychedelic therapy under study. Psilocybin was popularized as a psychedelic recreational drug in the 1970s and was classified as a Schedule I drug by the DEA. Research on psilocybin as a medical treatment was restricted until the 1990s because of the sociocultural fear of dependence on this drug. As of 2022, psilocybin is the most commonly researched psychedelic due to its safety and low potential for abuse and dependence. Clinical trials are being conducted at universities and there is evidence confirming the use of psilocybin in the treatment of depression, post-traumatic stress disorder (PTSD) and end of life anxiety.
MDMA-assisted psychotherapy is the use of prescribed doses of MDMA as an adjunct to psychotherapy sessions. Research suggests that MDMA-assisted psychotherapy for post-traumatic stress disorder (PTSD), including Complex PTSD, might improve treatment effectiveness. In 2017, a Phase II clinical trial led to "breakthrough therapy" designation by the US Food and Drug Administration (FDA) for potential use as a treatment for PTSD.
Psychoplastogens are a group of small molecule drugs that produce rapid and sustained effects on neuronal structure and function, intended to manifest therapeutic benefit after a single administration. Several existing psychoplastogens have been identified and their therapeutic effects demonstrated; several are presently at various stages of development as medications including ketamine, MDMA, scopolamine, and the serotonergic psychedelics, including LSD, psilocin, DMT, and 5-MeO-DMT. Compounds of this sort are being explored as therapeutics for a variety of brain disorders including depression, addiction, and PTSD. The ability to rapidly promote neuronal changes via mechanisms of neuroplasticity was recently discovered as the common therapeutic activity and mechanism of action.

Delix Therapeutics is an American biotech company based in Boston, Massachusetts. The company develops novel neuroplasticity-promoting therapeutics for central nervous system (CNS) diseases such as depression and post-traumatic stress disorder (PTSD). It was co-founded in 2019 by David E. Olson and Nick Haft.
Psychedelic treatments for trauma-related disorders are the use of psychedelic substances, either alone or used in conjunction with psychotherapy, to treat trauma-related disorders. Trauma-related disorders, such as post-traumatic stress disorder (PTSD), have a lifetime prevalence of around 8% in the US population. However, even though trauma-related disorders can hinder the everyday life of individuals with them, less than 50% of patients who meet criteria for PTSD diagnosis receive proper treatment. Psychotherapy is an effective treatment for trauma-related disorders. A meta-analysis of treatment outcomes has shown that 67% of patients who completed treatment for PTSD no longer met diagnostic criteria for PTSD. For those seeking evidence-based psychotherapy treatment, it is estimated that 22-24% will drop out of their treatment. In addition to psychotherapy, pharmacotherapy (medication) is an option for treating PTSD; however, research has found that pharmacotherapy is only effective for about 59% of patients. Although both forms of treatment are effective for many patients, high dropout rates of psychotherapy and treatment-resistant forms of PTSD have led to increased research in other possible forms of treatment. One such form is the use of psychedelics.

Robin Lester Carhart-Harris is a British psychopharmacologist who is Ralph Metzner Distinguished Professor in the Department of Neurology at the University of California, San Francisco. Previously, he founded and was Head of the Centre for Psychedelic Research at Imperial College London.
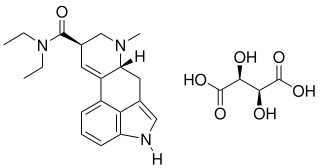
Lysergide d-tartrate (MM120) is an investigational new drug that was granted breakthrough therapy status for the treatment of Generalized Anxiety Disorder by the FDA in March 2024. It is a salt of lysergide.
A trip killer, or hallucinogen antidote, is a drug that aborts or reduces the effects of a hallucinogenic drug experience. As there are different types of hallucinogens that work in different ways, there are different types of trip killers. They can completely block or reduce the effects of hallucinogens or they can simply provide anxiety relief and sedation. Examples of trip killers, in the case of serotonergic psychedelics, include serotonin receptor antagonists, like antipsychotics and certain antidepressants, and benzodiazepines. Trip killers are sometimes used by recreational psychedelic users as a form of harm reduction to manage so-called bad trips, for instance difficult experiences with prominent anxiety. They can also be used clinically to manage effects of hallucinogens, like anxiety and psychomotor agitation, for instance in the emergency department.











