Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

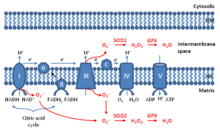
Superoxide dismutase (SOD, EC 1.15.1.1) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O−
2) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H
2O
2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is Lactobacillus plantarum and related lactobacilli, which use a different mechanism to prevent damage from reactive O−
2.
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
NADPH oxidase is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2.
Phenoptosis is a conception of the self-programmed death of an organism proposed by Vladimir Skulachev in 1999.

Superoxide dismutase [Cu-Zn] also known as superoxide dismutase 1 or hSod1 is an enzyme that in humans is encoded by the SOD1 gene, located on chromosome 21. SOD1 is one of three human superoxide dismutases. It is implicated in apoptosis, familial amyotrophic lateral sclerosis and Parkinson's disease.
Superoxide dismutase 2, mitochondrial (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD), is an enzyme which in humans is encoded by the SOD2 gene on chromosome 6. A related pseudogene has been identified on chromosome 1. Alternative splicing of this gene results in multiple transcript variants. This gene is a member of the iron/manganese superoxide dismutase family. It encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. Mutations in this gene have been associated with idiopathic cardiomyopathy (IDC), premature aging, sporadic motor neuron disease, and cancer.

Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the GPX1 gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans.
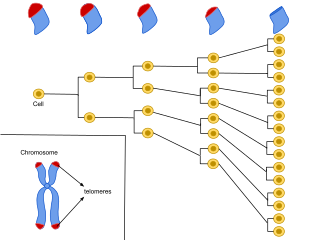
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.
Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. The process of mitophagy was first described over a hundred years ago by Margaret Reed Lewis and Warren Harmon Lewis. Ashford and Porter used electron microscopy to observe mitochondrial fragments in liver lysosomes by 1962, and a 1977 report suggested that "mitochondria develop functional alterations which would activate autophagy." The term "mitophagy" was in use by 1998.
All living cells produce reactive oxygen species (ROS) as a byproduct of metabolism. ROS are reduced oxygen intermediates that include the superoxide radical (O2−) and the hydroxyl radical (OH•), as well as the non-radical species hydrogen peroxide (H2O2). These ROS are important in the normal functioning of cells, playing a role in signal transduction and the expression of transcription factors. However, when present in excess, ROS can cause damage to proteins, lipids and DNA by reacting with these biomolecules to modify or destroy their intended function. As an example, the occurrence of ROS have been linked to the aging process in humans, as well as several other diseases including Alzheimer's, rheumatoid arthritis, Parkinson's, and some cancers. Their potential for damage also makes reactive oxygen species useful in direct protection from invading pathogens, as a defense response to physical injury, and as a mechanism for stopping the spread of bacteria and viruses by inducing programmed cell death.
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.
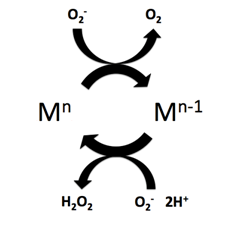
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme. SOD mimetics effectively convert the superoxide anion, a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase. Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases. SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.
Reductive stress (RS) is defined as an abnormal accumulation of reducing equivalents despite being in the presence of intact oxidation and reduction systems. A redox reaction involves the transfer of electrons from reducing agents (reductants) to oxidizing agents (oxidants) and redox couples are accountable for the majority of the cellular electron flow. RS is a state where there are more reducing equivalents compared to reductive oxygen species (ROS) in the form of known biological redox couples such as GSH/GSSG, NADP+/NADPH, and NAD+/NADH. Reductive stress is the counterpart to oxidative stress, where electron acceptors are expected to be mostly reduced. Reductive stress is likely derived from intrinsic signals that allow for the cellular defense against pro-oxidative conditions. There is a feedback regulation balance between reductive and oxidative stress where chronic RS induce oxidative species (OS), resulting in an increase in production of RS, again.

Perilipin 5, also known as Oxpatperilipin 5 or PLIN5, is a protein that belongs to perilipin family. This protein group has been shown to be responsible for lipid droplet's biogenesis, structure and degradation. In particular, Perilipin 5 is a lipid droplet-associated protein whose function is to keep the balance between lipolysis and lipogenesis, as well as maintaining lipid droplet homeostasis. For example, in oxidative tissues, muscular tissues and cardiac tissues, PLIN5 promotes association between lipid droplets and mitochondria.

The mitochondrial theory of ageing has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of ageing. It was formulated by J. Miquel and colleagues in 1980 and was developed in the works of Linnane and coworkers (1989). The second was proposed by A. N. Lobachev in 1978.