Related Research Articles
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
DNA glycosylases are a family of enzymes involved in base excision repair, classified under EC number EC 3.2.2. Base excision repair is the mechanism by which damaged bases in DNA are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond.
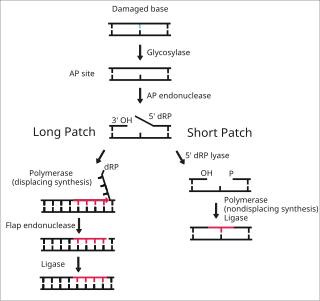
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky helix-distorting lesions. BER is important for removing damaged bases that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognize and remove specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease. The resulting single-strand break can then be processed by either short-patch or long-patch BER.
The adaptive response is a form of direct DNA repair in E. coli that protects DNA from damage by external agents or by errors during replication. It is initiated against alkylation, particularly methylation, of guanine or thymine nucleotides or phosphate groups on the sugar-phosphate backbone of DNA. Under sustained exposure to low-level treatment with alkylating mutagens, E. coli can adapt to the presence of the mutagen, rendering subsequent treatment with high doses of the same agent less effective.
Ada, also called as O6 alkyl guanine transferase I (O6 AGT I), is an enzyme induced by treatment of bacterial cells with alkylating agents that mainly cause methylation damage. This phenomenon is called the adaptive response hence the name. Ada transfers the alkyl group from DNA bases and sugar-phosphate backbone to a cysteine residue, inactivating itself. Consequently, it reacts stoichiometrically with its substrate rather than catalytically and is referred to as a suicide enzyme. Methylation of Ada protein converts it into a self transcriptional activator, inducing its own gene expression and the expression of other genes which together with Ada help the cells repair alkylation damage. Ada removes the alkyl group attached to DNA bases like guanine (O6-alkyl guanine) or thymine (O4-alkyl thymine) and to the oxygen of the phosphodiester backbone of the DNA. However, Ada shows greater preference for O6- alkyl guanine compared to either O4-thymine and alkylated phosphotriesters. Ada enzyme has two active sites, one for the alkylated guanines and thymines and the other for alkylated phosphotriesters.
AlkB (Alkylation B) is a protein found in E. coli, induced during an adaptive response and involved in the direct reversal of alkylation damage. AlkB specifically removes alkylation damage to single stranded (SS) DNA caused by SN2 type of chemical agents. It efficiently removes methyl groups from 1-methyl adenines, 3-methyl cytosines in SS DNA. AlkB is an alpha-ketoglutarate-dependent hydroxylase, a superfamily non-haem iron-containing proteins. It oxidatively demethylates the DNA substrate. Demethylation by AlkB is accompanied with release of CO2, succinate, and formaldehyde.
In enzymology, a methylated-DNA-[protein]-cysteine S-methyltransferase is an enzyme that catalyzes the chemical reaction

DNA-(apurinic or apyrimidinic site) lyase is an enzyme that in humans is encoded by the APEX1 gene.
The enzyme purine imidazole-ring cyclase (EC 4.3.2.4) catalyzes the chemical reaction

DNA-3-methyladenine glycosylase also known as 3-alkyladenine DNA glycosylase (AAG) or N-methylpurine DNA glycosylase (MPG) is an enzyme that in humans is encoded by the MPG gene.

Endonuclease III-like protein 1 is an enzyme that in humans is encoded by the NTHL1 gene.
Endonuclease VIII-like 1 is an enzyme that in humans is encoded by the NEIL1 gene.
Endonuclease VIII-like 2 is an enzyme that in humans is encoded by the NEIL2 gene.

The FPG IleRS zinc finger domain represents a zinc finger domain found at the C-terminal in both DNA glycosylase/AP lyase enzymes and in isoleucyl tRNA synthetase. In these two types of enzymes, the C-terminal domain forms a zinc finger.
In DNA repair, the Ada regulon is a set of genes whose expression is essential to adaptive response, which is triggered in prokaryotic cells by exposure to sub-lethal doses of alkylating agents. This allows the cells to tolerate the effects of such agents, which are otherwise toxic and mutagenic.

In molecular biology, the H2TH domain is a DNA-binding domain found in DNA glycosylase/AP lyase enzymes, which are involved in base excision repair of DNA damaged by oxidation or by mutagenic agents. Most damage to bases in DNA is repaired by the base excision repair pathway. These enzymes are primarily from bacteria, and have both DNA glycosylase activity EC 3.2.2.- and AP lyase activity EC 4.2.99.18. Examples include formamidopyrimidine-DNA glycosylases and endonuclease VIII (Nei).
DNA-3-methyladenine glycosylase I is an enzyme with systematic name alkylated-DNA glycohydrolase . This enzyme catalyses the following chemical reaction
DNA-3-methyladenine glycosylase II is an enzyme that catalyses the following chemical reaction:
Double-stranded uracil-DNA glycosylase is an enzyme with systematic name uracil-double-stranded DNA deoxyribohydrolase (uracil-releasing). This enzyme catalyses a specific chemical reaction: it hydrolyses mismatched double-stranded DNA and polynucleotides, releasing free uracil.

Christopher J. Chetsanga is a prominent Zimbabwean scientist who is a member of the African Academy of Sciences and The World Academy of Sciences. He discovered two enzymes involved in DNA repair. He has also held various academic administrative posts like Vice-Chancellor, Director and Dean.
References
- ↑ Boiteux S, O'Connor TR, Laval J (October 1987). "Formamidopyrimidine-DNA glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein". The EMBO Journal. 6 (10): 3177–83. PMC 553760 . PMID 3319582.
- ↑ Serre1; Pereira De Jésus, K; Boiteux, S; Zelwer, C; Castaing, B; et al. (2002). "Crystal structure of the Lactococcus lactis formamidopyrimidine-DNA glycosylase bound to an abasic site analogue-containing DNA". The EMBO Journal. 21 (12): 2854–2865. doi:10.1093/emboj/cdf304. PMC 126059 . PMID 12065399.
- ↑ Chetsanga C.J., Lindahl T. (1979). "Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli". Nucleic Acids Res. 6 (11): 3673–84. doi:10.1093/nar/6.11.3673. PMC 327965 . PMID 386277.