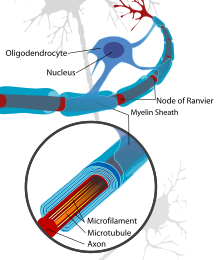
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and diploblasts. It is a structure composed of nervous tissue positioned along the rostral to caudal axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain, though precursor structures exist in onychophorans, gastropods and lancelets.

Myelin is a lipid-rich extramembranous structure found on the axons and dendrites of neuron in many bilaterian animals, mainly vertebrates, as well as some arthropods and annelids. A circumferential wrapping of myelin, known as a myelin sheath, increases the conduction speed of electrical impulses passing along the axon by generating saltatory conductions, which are much faster than conduction along an unmyelinated axon, and also reduce signal loss due to extrinsic disturbances.
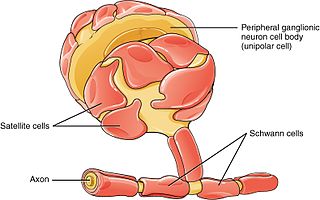
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.
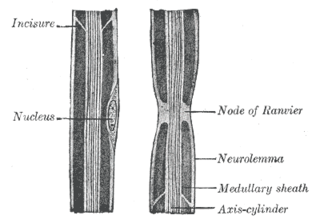
Neurilemma is the outermost nucleated cytoplasmic layer of Schwann cells that surrounds the axon of the neuron. It forms the outermost layer of the nerve fiber in the peripheral nervous system.
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.

Oligodendrocytes, also known as oligodendroglia, are a type of neuroglia whose main functions are to provide support and insulation to axons within the central nervous system (CNS) of jawed vertebrates. Their function is similar to that of Schwann cells, which perform the same task in the peripheral nervous system (PNS). Oligodendrocytes accomplish this by forming the myelin sheath around axons. Unlike Schwann cells, a single oligodendrocyte can extend its processes to cover around 50 axons, with each axon being wrapped in approximately 1 μm of myelin sheath. Furthermore, an oligodendrocyte can provide myelin segments for multiple adjacent axons.

In neuroscience and anatomy, nodes of Ranvier, also known as myelin-sheath gaps, occur along a myelinated axon where the axolemma is exposed to the extracellular space. Nodes of Ranvier are uninsulated and highly enriched in ion channels, allowing them to participate in the exchange of ions required to regenerate the action potential. Nerve conduction in myelinated axons is referred to as saltatory conduction due to the manner in which the action potential seems to "jump" from one node to the next along the axon. This results in faster conduction of the action potential.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.
Sulfatide, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebroside, is a class of sulfolipids, specifically a class of sulfoglycolipids, which are glycolipids that contain a sulfate group. Sulfatide is synthesized primarily starting in the endoplasmic reticulum and ending in the Golgi apparatus where ceramide is converted to galactocerebroside and later sulfated to make sulfatide. Of all of the galactolipids that are found in the myelin sheath, one fifth of them are sulfatide. Sulfatide is primarily found on the extracellular leaflet of the myelin plasma membrane produced by the oligodendrocytes in the central nervous system and in the Schwann cells in the peripheral nervous system. However, sulfatide is also present on the extracellular leaflet of the plasma membrane of many cells in eukaryotic organisms.

Myelin incisures are small pockets of cytoplasm left behind during the Schwann cell myelination process.
Remyelination is the process of propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. The process creates a thinner myelin sheath than normal, but it helps to protect the axon from further damage, from overall degeneration, and proves to increase conductance once again. The processes underlying remyelination are under investigation in the hope of finding treatments for demyelinating diseases, such as multiple sclerosis.

Myelin-associated glycoprotein is a type 1 transmembrane protein glycoprotein localized in periaxonal Schwann cell and oligodendrocyte membranes, where it plays a role in glial-axonal interactions. MAG is a member of the SIGLEC family of proteins and is a functional ligand of the NOGO-66 receptor, NgR. MAG is believed to be involved in myelination during nerve regeneration in the PNS and is vital for the long-term survival of the myelinated axons following myelinogenesis. In the CNS MAG is one of three main myelin-associated inhibitors of axonal regeneration after injury, making it an important protein for future research on neurogenesis in the CNS.
Neuroregeneration involves the regrowth or repair of nervous tissues, cells or cell products. Neuroregenerative mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.

Myelin protein zero is a single membrane glycoprotein which in humans is encoded by the MPZ gene. P0 is a major structural component of the myelin sheath in the peripheral nervous system (PNS). Myelin protein zero is expressed by Schwann cells and accounts for over 50% of all proteins in the peripheral nervous system, making it the most common protein expressed in the PNS. Mutations in myelin protein zero can cause myelin deficiency and are associated with neuropathies like Charcot–Marie–Tooth disease and Dejerine–Sottas disease.

Gap junction beta-1 protein (GJB1), also known as connexin 32 (Cx32), is a transmembrane protein that in humans is encoded by the GJB1 gene. Gap junction beta-1 protein is a member of the gap junction connexin family of proteins that regulates and controls the transfer of communication signals across cell membranes, primarily in the liver and peripheral nervous system. However, the protein is expressed in multiple organs, including in oligodendrocytes in the central nervous system.
In neurobiology, a mesaxon is a pair of parallel plasma membranes of a Schwann cell. It marks the point of edge-to-edge contact by the Schwann cell encircling the axon. A single Schwann cell of the peripheral nervous system will wrap around and support only one individual axon, while the oligodendrocytes found in the central nervous system can wrap around and support 5-8 axons. Thin unmyelinated axons are often bundled, with several unmyelinated axons to a single mesaxon.

Leucine rich repeat and Immunoglobin-like domain-containing protein 1 also known as LINGO-1 is a protein which is encoded by the LINGO1 gene in humans. It belongs to the family of leucine-rich repeat proteins which are known for playing key roles in the biology of the central nervous system. LINGO-1 is a functional component of the Nogo receptor also known as the reticulon 4 receptor.
Anti-MAG peripheral neuropathy is a specific type of peripheral neuropathy in which the person's own immune system attacks cells that are specific in maintaining a healthy nervous system. As these cells are destroyed by antibodies, the nerve cells in the surrounding region begin to lose function and create many problems in both sensory and motor function. Specifically, antibodies against myelin-associated glycoprotein (MAG) damage Schwann cells. While the disorder occurs in only 10% of those afflicted with peripheral neuropathy, people afflicted have symptoms such as muscle weakness, sensory problems, and other motor deficits usually starting in the form of a tremor of the hands or trouble walking. There are, however, multiple treatments that range from simple exercises in order to build strength to targeted drug treatments that have been shown to improve function in people with this type of peripheral neuropathy.