Related Research Articles

Myelin is a lipid-rich material that surrounds nerve cell axons to insulate them and increase the rate at which electrical impulses pass along the axon. The myelinated axon can be likened to an electrical wire with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, called nodes of Ranvier. At the nodes of Ranvier, which are approximately one thousandth of a mm in length, the axon's membrane is bare of myelin.

Choline acetyltransferase is a transferase enzyme responsible for the synthesis of the neurotransmitter acetylcholine. ChAT catalyzes the transfer of an acetyl group from the coenzyme acetyl-CoA to choline, yielding acetylcholine (ACh). ChAT is found in high concentration in cholinergic neurons, both in the central nervous system (CNS) and peripheral nervous system (PNS). As with most nerve terminal proteins, ChAT is produced in the body of the neuron and is transported to the nerve terminal, where its concentration is highest. Presence of ChAT in a nerve cell classifies this cell as a "cholinergic" neuron. In humans, the choline acetyltransferase enzyme is encoded by the CHAT gene.

Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:

Connexins (Cx), or gap junction proteins, are structurally related transmembrane proteins that assemble to form vertebrate gap junctions. An entirely different family of proteins, the innexins, forms gap junctions in invertebrates. Each gap junction is composed of two hemichannels, or connexons, which consist of homo- or heterohexameric arrays of connexins, and the connexon in one plasma membrane docks end-to-end with a connexon in the membrane of a closely opposed cell. The hemichannel is made of six connexin subunits, each of which consist of four transmembrane segments. Gap junctions are essential for many physiological processes, such as the coordinated depolarization of cardiac muscle, proper embryonic development, and the conducted response in microvasculature. Connexins also have non-channel dependant functions relating to cytoskeleton and cell migration. For these reasons, mutations in connexin-encoding genes can lead to functional and developmental abnormalities.

Myelin basic protein (MBP) is a protein believed to be important in the process of myelination of nerves in the nervous system. The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the velocity of axonal impulse conduction. MBP maintains the correct structure of myelin, interacting with the lipids in the myelin membrane.
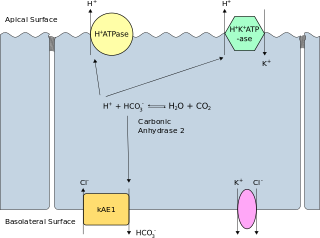
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
Molecular mimicry is the theoretical possibility that sequence similarities between foreign and self-peptides are enough to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. Despite the prevalence of several peptide sequences which can be both foreign and self in nature, just a few crucial residues can activate a single antibody or TCR. This highlights the importance of structural homology in the theory of molecular mimicry. Upon activation, these "peptide mimic" specific T or B cells can cross-react with self-epitopes, thus leading to tissue pathology (autoimmunity). Molecular mimicry is one of several ways in which autoimmunity can be evoked. A molecular mimicking event is more than an epiphenomenon despite its low probability, and these events have serious implications in the onset of many human autoimmune disorders.

Myelin-associated glycoprotein is a type 1 transmembrane protein glycoprotein localized in periaxonal Schwann cell and oligodendrocyte membranes, where it plays a role in glial-axonal interactions. MAG is a member of the SIGLEC family of proteins and is a functional ligand of the NOGO-66 receptor, NgR. MAG is believed to be involved in myelination during nerve regeneration in the PNS and is vital for the long-term survival of the myelinated axons following myelinogenesis. In the CNS MAG is one of three main myelin-associated inhibitors of axonal regeneration after injury, making it an important protein for future research on neurogenesis in the CNS.

Proteolipid protein 1 (PLP1) is a form of myelin proteolipid protein (PLP). Mutations in PLP1 are associated with Pelizaeus–Merzbacher disease. It is a 4 transmembrane domain protein which is proposed to bind other copies of itself on the extracellular side of the membrane. In a myelin sheath, as the layers of myelin wraps come together, PLP will bind itself and tightly hold the cellular membranes together.

Myelin protein zero is a single membrane glycoprotein which in humans is encoded by the MPZ gene. P0 is a major structural component of the myelin sheath in the peripheral nervous system (PNS). Myelin protein zero is expressed by Schwann cells and accounts for over 50% of all proteins in the peripheral nervous system, making it the most common protein expressed in the PNS. Mutations in myelin protein zero can cause myelin deficiency and are associated with neuropathies like Charcot–Marie–Tooth disease and Dejerine–Sottas disease.

Prosaposin, also known as PSAP, is a protein which in humans is encoded by the PSAP gene.
Myelinogenesis is the formation and development of myelin sheaths in the nervous system, typically initiated in late prenatal neurodevelopment and continuing throughout postnatal development. Myelinogenesis continues throughout the lifespan to support learning and memory via neural circuit plasticity as well as remyelination following injury. Successful myelination of axons increases action potential speed by enabling saltatory conduction, which is essential for timely signal conduction between spatially separate brain regions, as well as provides metabolic support to neurons.

Gap junction beta-1 protein (GJB1), also known as connexin 32 (Cx32), is a transmembrane protein that in humans is encoded by the GJB1 gene. Gap junction beta-1 protein is a member of the gap junction connexin family of proteins that regulates and controls the transfer of communication signals across cell membranes, primarily in the liver and peripheral nervous system. However, the protein is expressed in multiple organs, including in oligodendrocytes in the central nervous system.

Peripheral myelin protein 22 (PMP22), also called Growth arrest-specific protein 3 (GAS-3), is a protein which in humans is encoded by the PMP22 gene. Mutations in PMP22 cause changes in the expression of peripheral myelin protein 22 which can result in several neuropathies.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Glycoprotein Ib (platelet), beta polypeptide (GP1BB) also known as CD42c, is a protein that in humans is encoded by the GP1BB gene.

Glycophorin B (MNS blood group) (gene designation GYPB) also known as sialoglycoprotein delta and SS-active sialoglycoprotein is a protein which in humans is encoded by the GYPB gene. GYPB has also recently been designated CD235b (cluster of differentiation 235b).
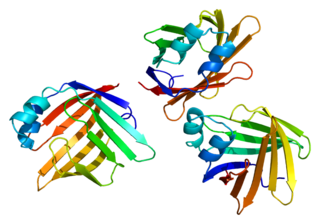
Myelin P2 protein is a protein that in humans is encoded by the PMP2 gene. Myelin protein P2 is a constituent of peripheral nervous system (PNS) myelin, also present in small amounts in central nervous system (CNS) myelin. As a structural protein, P2 is thought to stabilize the myelin membranes, and may play a role in lipid transport in Schwann cells. Structurally, P2 belongs to the family of cytoplasmic fatty acid-binding proteins (FABPs).

Synapsin I, is the collective name for Synapsin Ia and Synapsin Ib, two nearly identical phosphoproteins that in humans are encoded by the SYN1 gene. In its phosphorylated form, Synapsin I may also be referred to as phosphosynaspin I. Synapsin I is the first of the proteins in the synapsin family of phosphoproteins in the synaptic vesicles present in the central and peripheral nervous systems. Synapsin Ia and Ib are close in length and almost the same in make up, however, Synapsin Ib stops short of the last segment of the C-terminal in the amino acid sequence found in Synapsin Ia.
References
- ↑ Dautigny A, Popot JL, Pham Dinh D (1991). "Major Myelin proteolipid: the 4-alpha-helix topology". J. Membr. Biol. 120 (3): 233–246. doi:10.1007/BF01868534. PMID 1711121. S2CID 24450880.
- ↑ Kitamura K, Sakamoto Y, Yoshimura K, Nishijima T, Uyemura K (1987). "Complete amino acid sequence of PO protein in bovine peripheral nerve myelin". J. Biol. Chem. 262 (9): 4208–4214. doi: 10.1016/S0021-9258(18)61334-1 . PMID 2435734.
- ↑ Stoffel W, Schliess F (1991). "Evolution of the myelin integral membrane proteins of the central nervous system". Biol. Chem. Hoppe-Seyler. 372 (9): 865–874. doi:10.1515/bchm3.1991.372.2.865. PMID 1722981.
- ↑ Weimbs T, Sto ffel W (1992). "Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP". Biochemistry. 31 (49): 12289–12296. doi:10.1021/bi00164a002. PMID 1281423.
- ↑ Yan Y, Lagenaur C, Narayanan V (1993). "Molecular cloning of M6: identification of a PLP/DM20 gene family". Neuron. 11 (3): 423–431. doi:10.1016/0896-6273(93)90147-J. PMID 8398137. S2CID 46719251.