
Vulcanization is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides.
Accelerator may refer to:

An elastomer is a polymer with viscoelasticity and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of elastic polymer, is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation, without breaking of covalent bonds, is feasible. At ambient temperatures, such rubbers are thus relatively compliant and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and insulating elements. The importance of these rubbers can be judged from the fact that global revenues are forecast to rise to US$56 billion in 2020.
Accelerants are substances that can bond, mix or disturb another substance and cause an increase in the speed of a natural, or artificial chemical process. Accelerants play a major role in chemistry—most chemical reactions can be hastened with an accelerant. Accelerants alter a chemical bond, speed up a chemical process, or bring organisms back to homeostasis. Accelerants are not necessarily catalysts as they may be consumed by the process.
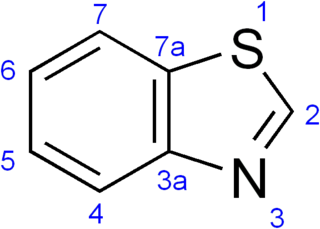
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.
Thiazolidine is a heterocyclic organic compound with the formula (CH2)3(NH)S. It is a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions. It is a sulfur analog of oxazolidine. Thiazolidine is a colorless liquid.

Pneumatic tires are manufactured according to relatively standardized processes and machinery, in around 455 tire factories in the world. With over 1 billion tires manufactured worldwide annually, the tire industry is a major consumer of natural rubber. Tire factories start with bulk raw materials such as synthetic rubber, carbon black, and chemicals and produce numerous specialized components that are assembled and cured.

Thiocarbanilide is an organic chemical compound with the formula (C6H5NH)2CS. This white solid is a derivative of thiourea. It is prepared by the reaction of aniline and carbon disulfide.

Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, it is a weak base, compared to strong bases such as NaOH, but it is a stronger base than its aromatic analog, aniline.
Rubber Technology is the subject dealing with the transformation of rubbers or elastomers into useful products, such as automobile tires, rubber mats and, exercise rubber stretching bands. The materials includes latex, natural rubber, synthetic rubber and other polymeric materials, such as thermoplastic elastomers. Rubber processed through such methods are components of a wide range of items.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents.

Pentanal (also called valeraldehyde) is the organic compound is an alkyl aldehyde, molecular formula C5H10O. It is used in flavorings, resin chemistry, and rubber accelerators. Its smell is described as fermented, bready, fruity, nutty, berry.

tert-Butylamine is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
David Spence was one of the pioneering rubber chemists. He helped the war effort during the Second World War by devising new ways of extracting natural rubbers from plants, and worked to improve the processing of the rubber. Over the course of his career, he worked to improve the dyeing processes for rubber products and the vulcanization of rubber, and in developing new accelerants for strengthening lower-quality natural rubber. In 1941, he became the first recipient of the Charles Goodyear Medal, awarded by the American Chemical Society.
Lorin Beryl Sebrell was an American scientist at the Goodyear Tire and Rubber Co. noted for identifying mercaptobenzothiazole as a vulcanization accelerator. In 1942, Sebrell received the Charles Goodyear Medal.
Aubert Y. Coran is an American scientist noted for his contributions to the development of rubber. In 1983, he won the Melvin Mooney Distinguished Technology Award, bestowed by the American Chemical Society to individuals "who have exhibited exceptional technical competency by making significant and repeated contributions to rubber science and technology". In 1995, the rubber division of the American Chemical Society bestowed on Coran the Charles Goodyear Medal in honor of his international contributions to polymer science and development.
2-Methyl-2-heptanethiol is an organic compound classified as a thiol. It is a straw-colored liquid with a strong, obnoxious odor.

2-Mercaptobenzothiazole is an organosulfur compound with the formula C
6H
4(NH)SC=S. It is used in the sulfur vulcanization of rubber.
In polymer chemistry, materials science, and food science, bloom refers to the migration of one component of a solid mixture to the surface of an article. The process is an example of phase separation or phase aggregation.

Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-based curatives or accelerators. Sulfur forms cross-linking bridges between sections of polymer chains which affects the mechanical and electronic properties. Many products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts. The term vulcanization is derived from Vulcan, the Roman god of fire.











