
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.
The term chloride refers to a compound or molecule that contains either a chlorine anion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. The pronunciation of the word "chloride" is.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.
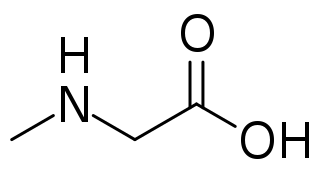
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.

In metallurgy, a flux is a chemical reducing agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Laundry detergent is a type of detergent used for cleaning dirty laundry (clothes). Laundry detergent is manufactured in powder and liquid form.
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst.
Tall oil, also called liquid rosin or tallol, is a viscous yellow-black odorous liquid obtained as a by-product of the kraft process of wood pulp manufacture when pulping mainly coniferous trees. The name originated as an anglicization of the Swedish tallolja. Tall oil is the third largest chemical by-product in a kraft mill after lignin and hemicellulose; the yield of crude tall oil from the process is in the range of 30–50 kg / ton pulp. It may contribute to 1.0–1.5% of the mill's revenue if not used internally.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.

Phenylsodium C6H5Na is an organosodium compound. Solid phenylsodium was first isolated by Nef in 1903. Although the behavior of phenylsodium and phenyl magnesium bromide are similar, the organosodium compound is very rarely used.

Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.
11-Aminoundecanoic acid is an organic compound with the formula H2N(CH2)10CO2H. This white solid is classified as an amine and a fatty acid. 11-Aminoundecanoic acid is a precursor to Nylon-11.

N,N,N′,N′-Tetramethylformamidinium chloride is the simplest representative of quaternary formamidinium cations of the general formula [R2N−CH=NR2]+ with a chloride as a counterion in which all hydrogen atoms of the protonated formamidine [HC(=NH2)NH2]+ are replaced by methyl groups.
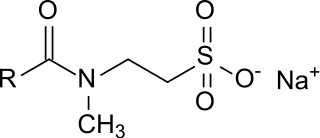
Taurates (or taurides) are a group of mild anionic surfactants. They are composed of a hydrophilic head group, consisting of N-methyltaurine (2-methylaminoethanesulfonic acid) and a lipophilic residue, consisting of a long-chain carboxylic acid (fatty acid), both linked via an amide bond. The fatty acids used could be lauric (C12), myristic (C14), palmitic (C16) or stearic acid (C18), but mainly mixtures of oleic acid (C18:1) and coconut fatty acid (C8 – C18) are used. Besides sodium, no other counterions play a relevant role (these could be e. g. ammonium or other alkali or alkaline earth metals).


















