
Acetylene (systematic name: ethyne) is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
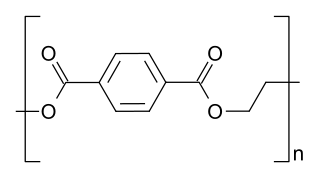
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers.

Polyvinylpyrrolidone (PVP), also commonly called polyvidone or povidone, is a water-soluble polymer made from the monomer N-vinylpyrrolidone.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.

Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCOO−. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities. Modified acrylates are also numerous, some examples being methacrylates (CH2=C(CH3)CO2R) and cyanoacrylates (CH2=C(CN)CO2R). Acrylate can also refer to polyacrylates prepared through the polymerization of the vinyl groups of acrylate monomers.

Propyne (methylacetylene) is an alkyne with the chemical formula CH3C≡CH. It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.
In chemistry, a trimer is a molecule or an anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.
Vinyl polymers are a group of polymers derived from substituted vinyl monomers of the type CH2=CHR. Their backbone is an extended alkane chain ...-CH2-CHR-CH2-CHR-..). In popular usage, "vinyl" refers only to polyvinyl chloride (PVC).

Vinylacetylene is the organic compound with the formula C4H4. The colourless gas was once used in the polymer industry. It is composed of both alkyne and alkene groups and is the simplest enyne.

In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.

N-Methyl-2-pyrrolidone (NMP) is an organic compound consisting of a 5-membered lactam. It is a colorless liquid, although impure samples can appear yellow. It is miscible with water and with most common organic solvents. It also belongs to the class of dipolar aprotic solvents such as dimethylformamide and dimethyl sulfoxide. It is used in the petrochemical and plastics industries as a solvent, exploiting its nonvolatility and ability to dissolve diverse materials.
2-Pyrrolidone, also known as 2-pyrrolidinone or butyrolactam, is an organic compound consisting of a 5-membered lactam, making it the simplest γ-lactam. It is a colorless liquid that is miscible with water and most common organic solvents.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.
Telomerization is a reaction that produces a particular kind of oligomer with two distinct end groups. The oligomer is called a telomer. Some telomerizations proceed by radical pathways, many do not. A generic equation is:

N-Vinylcarbazole is an organic compound used as a monomer in the production of poly(vinylcarbazole), a conductive polymer, in which conductivity is photon-dependent. The compound is used in the photoreceptors of photocopiers. Upon exposure to γ-irradiation, N-vinylcarbazole undergoes solid-state polymerisation.
Methyl vinyl ether is an organic compound with the chemical formula CH3OCH=CH2. A colorless gas, it is the simplest enol ether. It is used as a synthetic building block, as is the related compound ethyl vinyl ether (a liquid at room temperature).
Alkynylation is an addition reaction in organic synthesis where a terminal alkyne adds to a carbonyl group to form an α-alkynyl alcohol. When the acetylide is formed from acetylene, the reaction gives an α-ethynyl alcohol. This process is often referred to as ethynylation. Such process often involve metal acetylide intermediates
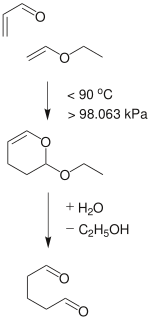
Ethyl vinyl ether is an organic compound with the chemical formula CH3CH2OCH=CH2. It is the simplest enol ether that is liquid at room temperature. It is used as a synthetic building block and a monomer.














