Related Research Articles

The British National Formulary (BNF) is a United Kingdom (UK) pharmaceutical reference book that contains a wide spectrum of information and advice on prescribing and pharmacology, along with specific facts and details about many medicines available on the UK National Health Service (NHS). Information within the BNF includes indication(s), contraindications, side effects, doses, legal classification, names and prices of available proprietary and generic formulations, and any other notable points. Though it is a national formulary, it nevertheless also includes entries for some medicines which are not available under the NHS, and must be prescribed and/or purchased privately. A symbol clearly denotes such drugs in their entry.

The Department of Health and Social Care (DHSC) is a ministerial department of the Government of the United Kingdom. It is responsible for government policy on health and adult social care matters in England, along with a few elements of the same matters which are not otherwise devolved to the Scottish Government, Welsh Government or Northern Ireland Executive. It oversees the English National Health Service (NHS). The department is led by the Secretary of State for Health and Social Care with three ministers of state and three parliamentary under-secretaries of state.

The National Institute for Health and Care Excellence (NICE) is an executive non-departmental public body, in England, of the Department of Health and Social Care, that publishes guidelines in four areas:
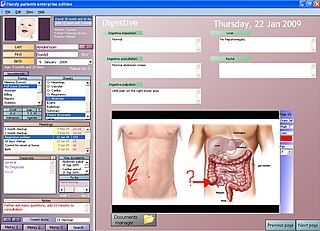
An electronic health record (EHR) is the systematized collection of patient and population electronically stored health information in a digital format. These records can be shared across different health care settings. Records are shared through network-connected, enterprise-wide information systems or other information networks and exchanges. EHRs may include a range of data, including demographics, medical history, medication and allergies, immunization status, laboratory test results, radiology images, vital signs, personal statistics like age and weight, and billing information.
Clinical audit is a process that has been defined as a quality improvement process that seeks to improve patient care and outcomes through systematic review of care against explicit criteria and the implementation of change
A special health authority is a type of NHS body which provide services on behalf of the National Health Service in England. Unlike other types of trust, they operate nationally rather than serve a specific geographical area.
Professor Sir Bruce Edward Keogh, KBE, FMedSci, FRCS, FRCP is a Rhodesian-born British surgeon who specialises in cardiac surgery. He was medical director of the National Health Service in England from 2007 and national medical director of the NHS Commissioning Board from 2013 until his retirement early in 2018. He is chair of Birmingham Women's and Children's NHS Foundation Trust and chairman of The Scar Free Foundation.
A patient safety organization (PSO) is a group, institution, or association that improves medical care by reducing medical errors. Common functions of patient safety organizations are data collection, analysis, reporting, education, funding, and advocacy. A PSO differs from a Federally designed Patient Safety Organization (PSO), which provides health care providers in the U.S. privilege and confidentiality protections for efforts to improve patient safety and the quality of patient care delivery

Ara Warkes Darzi, Baron Darzi of Denham is an Armenian-British surgeon, academic, and politician.
The Centre for Reviews and Dissemination (CRD) is a health services research centre based at the University of York, England. CRD was established in January 1994, and aims to provide research-based information for evidence-based medicine. CRD carries out systematic reviews and meta-analyses of healthcare interventions, and disseminates the results of research to decision-makers in the NHS.
The Health and Social Care Select Committee is a Departmental Select Committee of the British House of Commons, the lower house of the United Kingdom Parliament. Its remit is to examine the policy, administration and expenditure of the Department of Health and Social Care (DHSC) and its associated agencies and public bodies. The Clerks of the Committee are Previn Desai and Joanna Dodd.
The National electronic Library for Health (NeLH) was a digital library service provided by the NHS for healthcare professionals and the public between 1998 and 2006. It briefly became the National Library for Health and elements of it continue to this day as NHS Evidence, managed by the National Institute for Health and Care Excellence, and a range of services provided by Health Education England's Library and Knowledge Service Leads.

The National Health Service (NHS) is the publicly funded healthcare system in England, and one of the four National Health Service systems in the United Kingdom. It is the second largest single-payer healthcare system in the world after the Brazilian Sistema Único de Saúde. Primarily funded by the government from general taxation, and overseen by the Department of Health and Social Care, the NHS provides healthcare to all legal English residents and residents from other regions of the UK, with most services free at the point of use for most people. The NHS also conducts research through the National Institute for Health and Care Research (NIHR).
Healthcare in England is mainly provided by the National Health Service (NHS), a public body that provides healthcare to all permanent residents in England, that is free at the point of use. The body is one of four forming the UK National Health Service, as health is a devolved matter; there are differences with the provisions for healthcare elsewhere in the United Kingdom, and in England it is overseen by NHS England. Though the public system dominates healthcare provision in England, private health care and a wide variety of alternative and complementary treatments are available for those willing and able to pay.
The National Institute for Health and Care Research (NIHR) is the British government's major funder of clinical, public health, social care and translational research. With a budget of over £1.2 billion in 2020–21, its mission is to "improve the health and wealth of the nation through research". The NIHR was established in 2006 under the government's Best Research for Best Health strategy, and is funded by the Department of Health and Social Care. As a research funder and research partner of the NHS, public health and social care, the NIHR complements the work of the Medical Research Council. NIHR focuses on translational research, clinical research and applied health and social care research.

The National Collaborating Centre for Mental Health (NCCMH) is a collaboration between the Royal College of Psychiatrists and the Centre for Outcomes Research and Effectiveness at University College London (UCL). The NCCMH aims to promote the role of evidence synthesis in making informed judgments about healthcare policy. The NCCMH has a history of developing guidelines, conducting systematic reviews and developing implementation guidance for commissioners and service providers. Formed in 2001, on 1 April 2016 a new guideline development centre, the National Guideline Alliance, based at the Royal College of Obstetricians and Gynaecologists took over the clinical guideline programme that had been run by NCCMH.
Healthcare Improvement Scotland (HIS) is the national healthcare improvement organisation for Scotland. It is a public body which is part of the Scottish National Health Service, created in April 2011.

The Health and Social Care Act 2012 is an act of the Parliament of the United Kingdom. It provided for the most extensive reorganisation of the structure of the National Health Service in England to date. It removed responsibility for the health of citizens from the Secretary of State for Health, which the post had carried since the inception of the NHS in 1948. It abolished primary care trusts (PCTs) and strategic health authorities (SHAs) and transferred between £60 billion and £80 billion of "commissioning", or healthcare funds, from the abolished PCTs to several hundred clinical commissioning groups, partly run by the general practitioners (GPs) in England. A new executive agency of the Department of Health, Public Health England, was established under the act on 1 April 2013.
The Cancer Drugs Fund (CDF) was introduced in England in 2011. It was established in order to provide a means by which National Health Service (NHS) patients in England could receive cancer drugs that had been rejected by National Institute for Health and Care Excellence because they were not cost effective. Its establishment was confirmed by the UK government's coalition agreement in 2010, and by the White Paper, Equity and excellence – Liberating the NHS.
Health Education England (HEE) is an executive non-departmental public body of the Department of Health and Social Care. Its function is to provide national leadership and coordination for the education and training within the health and public health workforce within England. It has been operational since June 2012.
References
- ↑ "About Us". National Institute for Health and Care Excellence. 30 August 2024. Archived from the original on 23 May 2013. Retrieved 30 August 2024.
- 1 2 "Evidence search service closure information". National Institute for Health and Care Excellence. 2024.
- ↑ "High Quality Care For All NHS Next Stage Review Final Report" (PDF).
- 1 2 "Our NHS Our Future: NHS Next Stage Review Leading Local Change". Department of Health. May 2008. 084644
- 1 2 "UK Government Web Archive". webarchive.nationalarchives.gov.uk. Retrieved 27 September 2023.
- ↑ "NICE Evidence Search" (PDF).
- ↑ "Ethnicity and Health". NICE. Archived from the original on 16 February 2007.
- ↑ "Budget for NHS Evidence - a Freedom of Information request to National Institute for Health and Clinical Excellence". 16 March 2010.
- ↑ "NICE Electronic & Print Content Framework". NICE. Retrieved 4 June 2013.
- ↑ "About NICE International | NICE International | What we do | About". NICE. Retrieved 31 August 2024.
- ↑ Cheng, Tsung-Mei (15 September 2009). "Nice approach". Financial Times . Retrieved 18 September 2009.
- ↑ "Annual General Meeting and Public Board Meeting" (PDF). National Institute for Health and Care Excellence. 19 July 2017. Archived (PDF) from the original on 21 January 2022. Retrieved 15 June 2022.