
A compressed-air car is a compressed-air vehicle powered by pressure vessels filled with compressed air. It is propelled by the release and expansion of the air within a motor adapted to compressed air. The car might be powered solely by air, or combined with other fuels such as gasoline, diesel, or an electric plant with regenerative braking.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold. In late 2024 global demand passed 1 Terawatt-hour per year, while production capacity was more than twice that.

A rechargeable battery, storage battery, or secondary cell, is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer.

A lithium polymer battery, or more correctly, lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. Highly conductive semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types. They are used in applications where weight is critical, such as mobile devices, radio-controlled aircraft, and some electric vehicles.

A sodium–sulfur (NaS) battery is a type of molten-salt battery that uses liquid sodium and liquid sulfur electrodes. This type of battery has a similar energy density to lithium-ion batteries, and is fabricated from inexpensive and low-toxicity materials. Due to the high operating temperature required, as well as the highly reactive nature of sodium and sodium polysulfides, these batteries are primarily suited for stationary energy storage applications, rather than for use in vehicles. Molten Na-S batteries are scalable in size: there is a 1 MW microgrid support system on Catalina Island CA (USA) and a 50 MW/300 MWh system in Fukuoka, Kyushu, (Japan). In 2024, only one company produced molten NaS batteries on a commercial scale. BASF Stationary Energy Storage GmbH, a wholly owned subsidiary of BASF SE, acts as a distributor and development partner for the NaS batteries produced by NGK Insulators.

A compressed-air vehicle (CAV) is a transport mechanism fueled by tanks of pressurized atmospheric gas and propelled by the release and expansion of the gas within a pneumatic motor.

The nickel–iron battery is a rechargeable battery having nickel(III) oxide-hydroxide positive plates and iron negative plates, with an electrolyte of potassium hydroxide. The active materials are held in nickel-plated steel tubes or perforated pockets. It is a very robust battery which is tolerant of abuse, and can have very long life even if so treated. It is often used in backup situations where it can be continuously charged and can last for more than 20 years. Due to its low specific energy, poor charge retention, and high cost of manufacture, other types of rechargeable batteries have displaced the nickel–iron battery in most applications.
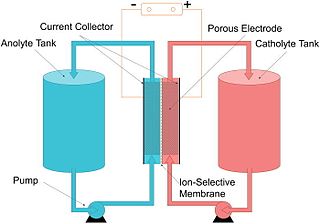
The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery. It employs vanadium ions as charge carriers. The battery uses vanadium's ability to exist in a solution in four different oxidation states to make a battery with a single electroactive element instead of two. For several reasons, including their relative bulkiness, vanadium batteries are typically used for grid energy storage, i.e., attached to power plants/electrical grids.

A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs across the membrane while the liquids circulate in their respective spaces.

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehiclesand for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.

A solar car is a solar vehicle for use on public roads or race tracks. Solar vehicles are electric vehicles that use self-contained solar cells to provide full or partial power to the vehicle via sunlight. Solar vehicles typically contain a rechargeable battery to help regulate and store the energy from the solar cells and from regenerative braking. Some solar cars can be plugged into external power sources to supplement the power of sunlight used to charge their battery.

An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach to the positive terminal, thus cause a redox reaction by attracting positively charged ions, cations. Thus converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.

A solid-state battery (SSB) is an electrical battery that uses a solid electrolyte for ionic conductions between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries. Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.

A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than solid-state capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries.
A hydrogen–bromine battery is a rechargeable flow battery in which hydrogen bromide (HBr) serves as the system’s electrolyte. During the charge cycle, as power flows into the stack, H2 is generated and stored in a separate tank, the other product of the chemical reaction is HBr3 which accumulates in the electrolyte. During the discharge cycle the H2 is combined again with the HBr3 and the system returns to its initial stage with a full tank of HBr. The electrolyte suffers no degradation during the process and the system is self contained with no emissions.
A lithium-ion flow battery is a flow battery that uses a form of lightweight lithium as its charge carrier. The flow battery stores energy separately from its system for discharging. The amount of energy it can store is determined by tank size; its power density is determined by the size of the reaction chamber.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.














