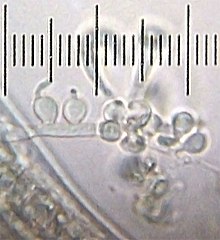
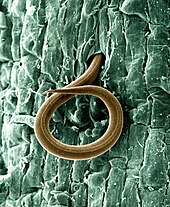
Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of Ascomycota are asexual and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.

A hypha is a long, branching, filamentous structure of a fungus, oomycete, or actinobacterium. In most fungi, hyphae are the main mode of vegetative growth, and are collectively called a mycelium.
Entomopathogenic fungi are parasitic unicellular or multicellular microorganisms belonging to the kingdom of Fungi, that can infect and seriously disable or kill insects. Entomopathogenicity is not limited to a particular class of fungi and is found in six divisions in the fungal kingdom. Some fungal entomopathogens are opportunistic whereas some have evolved into highly specific pathogens of insects.

Coprinus comatus, commonly known as the shaggy ink cap, lawyer's wig, or shaggy mane, is a common fungus often seen growing on lawns, along gravel roads and waste areas. The young fruit bodies first appear as white cylinders emerging from the ground, then the bell-shaped caps open out. The caps are white, and covered with scales—this is the origin of the common names of the fungus. The gills beneath the cap are white, then pink, then turn black and deliquesce ('melt') into a black liquid filled with spores. This mushroom is unusual because it will turn black and dissolve itself in a matter of hours after being picked or depositing spores.
This is a glossary of some of the terms used in phytopathology.
Hyphomycetes are a form classification of fungi, part of what has often been referred to as fungi imperfecti, Deuteromycota, or anamorphic fungi. Hyphomycetes lack closed fruit bodies, and are often referred to as moulds. Most hyphomycetes are now assigned to the Ascomycota, on the basis of genetic connections made by life-cycle studies or by phylogenetic analysis of DNA sequences; many remain unassigned phylogenetically.

The Pleurotaceae are a family of small to medium-sized mushrooms which have white spores. The family contains 13 genera over 412 species. Members of Pleurotaceae can be mistaken for members of Marasmiaceae. Perhaps the best known member is the oyster mushroom, Pleurotus ostreatus.

The Orbiliaceae are a family of saprobic sac fungi. It is the only family in the monotypic class Orbiliomycetes and the monotypic order Orbiliales. The family was first described by John Axel Nannfeldt in 1932 and now contains 288 species in 12 genera. Members of this family have a widespread distribution, but are more prevalent in temperate regions. Some species in the Orbiliaceae are carnivorous fungi, and have evolved a number of specialized mechanisms to trap nematodes.

Purpureocillium lilacinum is a species of filamentous fungus in the family Ophiocordycipitaceae. It has been isolated from a wide range of habitats, including cultivated and uncultivated soils, forests, grassland, deserts, estuarine sediments and sewage sludge, and insects. It has also been found in nematode eggs, and occasionally from females of root-knot and cyst nematodes. In addition, it has frequently been detected in the rhizosphere of many crops. The species can grow at a wide range of temperatures – from 8 to 38 °C for a few isolates, with optimal growth in the range 26 to 30 °C. It also has a wide pH tolerance and can grow on a variety of substrates. P. lilacinum has shown promising results for use as a biocontrol agent to control the growth of destructive root-knot nematodes.

Zoophagus is a genus of zygomycete fungi that preys on rotifers and nematodes. It was established in 1911 by Sommerstorff, who originally considered it to be an oomycete. It is common in a variety of freshwater habitats, such as ponds and sewage treatment plants.
Charles Frank Drechsler was an American mycologist with 45 years of research with the United States Department of Agriculture. He spent considerable time working with cereal fungal diseases, and the genus Drechslera was named after him. Drechsler also worked extensively on oomycete fungi and their interactions with vegetable plants. Drechsler was recognized as a leading authority on helminthosporia, oomycetes, and other parasitic fungi.

Harposporium anguillulae is a member of the genus Harposporium. It is an endoparasitic nematophagous fungus that attacks nematodes and eelworms and is isolated commonly from field and agricultural soils as well as used as an experimental organism in the laboratory.

Arthrobotrys oligospora was discovered in Europe in 1850 by Georg Fresenius. A. oligospora is the model organism for interactions between fungi and nematodes. It is the most common nematode-capturing fungus, and most widespread nematode-trapping fungus in nature. It was the first species of fungi documented to actively capture nematodes.
Dactylellina haptotyla is a common soil-living fungus that develops structures to capture nematodes as nutrient source. In the presence of nematodes, spores can germinate into sticky knobs or non-constricting loops. The fungus traps nematodes with sticky knobs and non-constricting loops, then breakdown the cuticle, and penetrates the body of nematodes to obtain nutrients. For its predatory nature, Dactylellina haptotyla is also considered as nematode-trapping fungus or carnivorous fungus.
Arthrobotrys dactyloides is a species of fungus in the family Orbiliaceae. It is nematophagous, forming loops of hypha to trap nematodes, on which it then feeds.
Stylopage is a polytypic genus of predacious fungus in the order Zoopagales, within the subphylum Zoopagomycotina. All known species of Stylopage subsist on various species of amoebae or nematodes by trapping their prey, typically using an adhesive substance that coats their vegetative hyphae, and absorbing nutrients through the projection of a haustorium. 17 extant Stylopage species have been described thus far.
Birgit Ann-Marie Margareta Nordbring-Hertz, was a Swedish scientist at Lund University known for her work on the interactions between fungi and nematodes.

Funneliformis mosseae is a species of fungus in the family Glomeraceae, which is an arbuscular mycorrhizal (AM) fungi that forms symbiotic relationships with plant roots. Funneliformis mosseae has a wide distribution worldwide, and can be found in North America, South America, Europe, Africa, Asia and Australia. Funneliformis are characterized by having an easily visible septum in the area of the spore base and are often cylindrical or funnel-shaped. Funneliformis mosseae similarly resembles Glomus caledonium, however the spore wall of Funneliformis mosseae contains three layers, whereas Gl. caledonium spore walls are composed of four layers. Funneliformis is an easily cultivated species which multiplies well in trap culture, along with its high distribution, F. mosseae is not considered endangered and is often used for experimental purposes when combined with another host.
Meristacrum is a fungal genus in the monotypic family Meristacraceae, of the order Entomophthorales. They are parasites of soil invertebrates, they typically infect nematodes, and tardigrades.
Arthrobotrys musiformis is a species of nematode catching fungi, genus Arthrobotrys. This, like other Arthrobotrys species, captures and feeds on nematodes. It is widespread, with its initial discovery being in Norfolk, Virginia. This species demonstrates promising anti-helminth potential, and is hypothesized to reduce the number of parasitic nematodes in plants and livestock as either a biocontrol or through isolating metabolites.