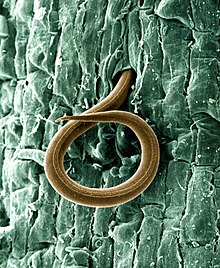
Northern root-knot nematode is a species of vegetable pathogens which produces tiny galls on around 550 crop and weed species. They invade root tissue after birth. Females are able to lay up to 1,000 eggs at a time in a large egg mass. By surviving harsh winters, they can survive in cold climates.

Meloidogyne incognita, also known as the southern root-nematode or cotton root-knot nematode is a plant-parasitic roundworm in the family Heteroderidae. This nematode is one of the four most common species worldwide and has numerous hosts. It typically incites large, usually irregular galls on roots as a result of parasitism.

Rotylenchulus reniformis, the reniform nematode, is a species of parasitic nematode of plants with a worldwide distribution in the tropical and subtropical regions.

Meloidogyne arenaria is a species of plant pathogenic nematodes. This nematode is also known as the peanut root knot nematode. The word "Meloidogyne" is derived from two Greek words that mean "apple-shaped" and "female". The peanut root knot nematode, M. arenaria is one of the "major" Meloidogyne species because of its worldwide economic importance. M. arenaria is a predominant nematode species in the United States attacking peanut in Alabama, Florida, Georgia, and Texas. The most damaging nematode species for peanut in the USA is M. arenaria race 1 and losses can exceed 50% in severely infested fields. Among the several Meloidogyne species that have been characterized, M. arenaria is the most variable both morphologically and cytologically. In 1949, two races of this nematode had been identified, race 1 which reproduces on peanut and race 2 which cannot do so. However, in a recent study, three races were described. López-Pérez et al (2011) had also studied populations of M. arenaria race 2, which reproduces on tomato plants carrying the Mi gene and race 3, which reproduces on both resistant pepper and tomato.

Meloidogyne javanica is a species of plant-pathogenic nematodes. It is one of the tropical root-knot nematodes and a major agricultural pest in many countries. It has many hosts. Meloidogyne javanica reproduces by obligatory mitotic parthenogenesis (apomixis).
Pratylenchus brachyurus is a plant parasitic nematode.

Pratylenchus penetrans is a species of nematode in the genus Pratylenchus, the lesion nematodes. It occurs in temperate regions worldwide, regions between the subtropics and the polar circles. It is an animal that inhabits the roots of a wide variety of plants and results in necrotic lesions on the roots. Symptoms of P. penetrans make it hard to distinguish from other plant pathogens; only an assay of soil can conclusively diagnose a nematode problem in the field. P. penetrans is physically very similar to other nematode species, but is characterized by its highly distinctive mouthpiece. P. penetrans uses its highly modified mouth organs to rupture the outer surface of subterranean plant root structures. It will then enter into the root interior and feed on the plant tissue inside. P. penetrans is considered to be a crop parasite and farmers will often treat their soil with various pesticides in an attempt to eliminate the damage caused by an infestation. In doing this, farmers will also eliminate many of the beneficial soil fauna, which will lead to an overall degradation of soil quality in the future. Alternative, more environmentally sustainable methods to control P. penetrans populations may be possible in certain regions.
Heterodera carotae is a plant pathogenic nematode commonly known as the carrot root nematode or carrot cyst nematode. It is found in Europe, Cyprus and India and is considered an invasive species in the United States. It causes damage to carrot crops and is very specific in its choice of hosts, only infecting Daucus carota and Daucus pulcherrima.
Hirschmanniella oryzae, i.e. rice root nematode (RRN), is among the major pests of rice and is the most common plant-parasitic nematode found on irrigated rice. Recent modifications in cultivation practices have led to a substantial increase in rice production, which has been accompanied by heightened levels of RRN. The proportional increases in RRN with rice production can be explained by the nematode's impeccable adaptation towards constantly flooded conditions in which irrigated rice is often being grown.
Meloidogyne brevicauda is a plant-parasitic nematode. It is also called tea root-knot nematode, mature tea nematode or Indian root-knot nematode. It is a member of the root-knot nematodes, which was identified by C. A. Loos in 1953 in Sri Lanka.
Xiphinema americanum, the American dagger nematode, is a species of plant pathogenic nematodes. It is one of many species that belongs to the genus Xiphinema. It was first described by N. A. Cobb in 1913, who found it on both sides of the United States on the roots of grass, corn, and citrus trees. Not only is Xiphinema americanum known to vector plant viruses, but also X. americanum has been referred to as "the most destructive plant parasitic nematode in America", and one of the four major nematode pests in the Southeastern United States.
Tylenchulus semipenetrans, also known as the citrus nematode or citrus root nematode, is a species of plant pathogenic nematodes and the causal agent of slow decline of citrus. T. semipenetrans is found in most citrus production areas and diverse soil textures worldwide. Their feeding strategy is semi-endoparasitic and has a very narrow host range among commonly grown crops. These nematodes are considered as major plant-parasitic nematode because they can cause 10-30% losses reported on citrus trees. They also parasitize other hosts such as olive, grape, persimmon and lilac. The citrus nematode was first discovered in California in 1913 by J. R. Hodges, a horticultural inspector for Los Angeles County, and was later described and named by Nathan Cobb that year. T. semipenetrans is the only species of Tylenchulidae that are economically important to agriculture.
Mesocriconema xenoplax is a species of plant parasitic nematodes. Nematodes of this particular species are collectively called ring nematodes.
There are many plant-parasitic species in the root-knot nematode genus (Meloidogyne) that attack coffee such as M. incognita, M. arenaria, M. exigua, M. javanica and M. coffeicola. Study has already shown interspecific variability coffee, in which show how this species can be adapting to new hosts and environments.

Meloidogyne enterolobii was originally described from a population collected from the pacara earpod tree in China in 1983. In 2001 it was reported for the first time in the continental USA in Florida. M. enterolobii is now considered one of the most important root-knot nematode species because of its ability of reproducing on root-knot nematode-resistant bell pepper and other economically important crops.

Purpureocillium lilacinum is a species of filamentous fungus in the family Ophiocordycipitaceae. It has been isolated from a wide range of habitats, including cultivated and uncultivated soils, forests, grassland, deserts, estuarine sediments and sewage sludge, and insects. It has also been found in nematode eggs, and occasionally from females of root-knot and cyst nematodes. In addition, it has frequently been detected in the rhizosphere of many crops. The species can grow at a wide range of temperatures – from 8 to 38 °C for a few isolates, with optimal growth in the range 26 to 30 °C. It also has a wide pH tolerance and can grow on a variety of substrates. P. lilacinum has shown promising results for use as a biocontrol agent to control the growth of destructive root-knot nematodes.
Heterodera zeae, the corn cyst nematode (CCN), is a plant parasitic nematode that feeds on Zea mays (maize/corn). The CCN has a limited economic impact worldwide due to its high soil temperature requirements.
Pasteuria is a genus of mycelial and endospore-forming, nonmotile gram-positive bacteria that are obligate parasites of some nematodes and crustaceans. The genus of Pasteuria was previously classified within the family Alicyclobacillaceae, but has since been moved to the family Pasteuriaceae.
Mononchoides fortidens, of the order Diplogasterida, is a free-living predacious nematode that feeds on both nematodes and bacteria . The predatory behavior of this nematode presents the opportunity to use it as a bio-control agent against other plant parasitic nematodes. It has been shown to have a preference for the second stage juveniles of Meloidogyne incognita.

Allodiplogaster sudhausi is a free-living nematode species in the Diplogastridae family. It was described in 2008 as Koerneria sudhausi, before being moved to the genus Allodiplogaster in 2014. A. sudhausi is omnivorous. It predates on other nematodes, but can be cultured on Escherichia coli OP50 bacterium on agar.