
Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, transmitted only between humans via the respiratory route, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.
Pseudoviridae is a family of viruses, which includes three genera.

Nodaviridae is a family of nonenveloped positive-strand RNA viruses. Vertebrates and invertebrates serve as natural hosts. Diseases associated with this family include: viral encephalopathy and retinopathy in fish. There are nine species in the family, assigned to two genera.

Orbivirus is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Sedoreovirinae. Unlike other reoviruses, orbiviruses are arboviruses. They can infect and replicate within a wide range of arthropod and vertebrate hosts. Orbiviruses are named after their characteristic doughnut-shaped capsomers.
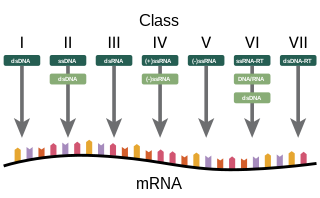
Baltimore classification is a system used to classify viruses based on their manner of messenger RNA (mRNA) synthesis. By organizing viruses based on their manner of mRNA production, it is possible to study viruses that behave similarly as a distinct group. Seven Baltimore groups are described that take into consideration whether the viral genome is made of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), whether the genome is single- or double-stranded, and whether the sense of a single-stranded RNA genome is positive or negative.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.

Alphanodavirus is a genus of non-enveloped positive-strand RNA viruses in the family Nodaviridae. Insects, mammals, and fishes serve as natural hosts. Diseases associated with this genus include: Nodamura virus paralysis in infected wax moth larvae. Member viruses can also provoke paralysis and death to suckling mice and suckling hamsters. There are five species in this genus.
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.
Lily virus X (LVX) is a pathogenic ssRNA(+) plant virus of the family Alphaflexiviridae and the order Tymovirales.
Entebbe bat virus is an infectious disease caused by a Flavivirus that is closely related to yellow fever.
Flock House virus (FHV) is in the alphanodavirus genus of the Nodaviridae family of viruses. Flock House virus was isolated from a grass grub at the Flock House research station in Bulls, New Zealand. FHV is an extensively studied virus and is considered a model system for the study of other non-enveloped RNA viruses owing to its small size and genetic tractability, particularly to study the role of the transiently exposed hydrophobic gamma peptide and the metastability of the viral capsid. FHV can be engineered in insect cell culture allowing for the tailored production of native or mutant authentic virions or virus-like-particles. FHV is a platform for nanotechnology and nanomedicine, for example, for epitope display and vaccine development. Viral entry into host cells occurs via receptor-mediated endocytosis. Receptor binding initiates a sequence of events during which the virus exploits the host environment in order to deliver the viral cargo in to the host cytosol. Receptor binding prompts the meta-stability of the capsid–proteins, the coordinated rearrangements of which are crucial for subsequent steps in the infection pathway. In addition, the transient exposure of a covalently-independent hydrophobic γ-peptide is responsible for breaching cellular membranes and is thus essential for the viral entry of FHV into host cells.

Middelburg virus (MIDV) is an alphavirus of the Old World Group that has likely endemic and zoonotic potential. It is of the viral family Togaviridae. It was isolated from mosquitos in 1957 in South Africa, MDIV antigens have now been found in livestock, horses, and humans.

The black queen cell virus (BQCV) is a virus that infects honey bees, specifically Apis mellifera, Apis florea, and Apis dorsata. Infection of the latter two species is more recent and can be attributed to genetic similarity and geographical closeness. It is important to learn about this virus because it is one of the most common bee viruses and bees are the most important pollinators. The agricultural industry depends on the bee's pollination to increase its economic value.
Black beetle virus (BBV) is a virus that was initially discovered in the North Island of New Zealand in Helensville in dead New Zealand black beetles in 1975.

Sepik virus (SEPV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus and family Flaviviridae. Flaviviridae is one of the most well characterized viral families, as it contains many well-known viruses that cause diseases that have become very prevalent in the world, like Dengue virus. The genus Flavivirus is one of the largest viral genera and encompasses over 50 viral species, including tick and mosquito borne viruses like Yellow fever virus and West Nile virus. Sepik virus is much less well known and has not been as well-classified as other viruses because it has not been known of for very long. Sepik virus was first isolated in 1966 from the mosquito Mansoniaseptempunctata, and it derives its name from the Sepik River area in Papua New Guinea, where it was first found. The geographic range of Sepik virus is limited to Papua New Guinea, due to its isolation.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.












