Nicotine is a naturally produced alkaloid in the nightshade family of plants and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits where it acts as a receptor antagonist.
Cotinine is an alkaloid found in tobacco plant and is also the predominant metabolite of nicotine. An anagram of the word "nicotine", it is used as a biomarker for exposure to tobacco smoke. Cotinine is currently being studied as a treatment for depression, PTSD, schizophrenia, Alzheimer's disease and Parkinson's disease. Cotinine was developed as an antidepressant as a fumaric acid salt, cotinine fumarate, to be sold under the brand name Scotine but it was never marketed.

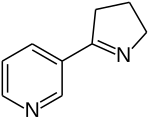
Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

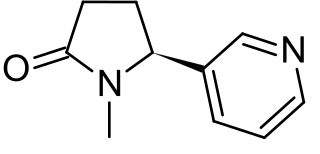
N-Nitrosonornicotine (NNN) is a tobacco-specific nitrosamine produced during the curing and processing of tobacco.

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy, euphoria and relaxation. Its psychoactive effects are comparable to that of nicotine.

Smokeless tobacco is a tobacco product that is used by means other than smoking. Their use involves chewing, sniffing, or placing the product between gum and the cheek or lip. Smokeless tobacco products are produced in various forms, such as chewing tobacco, snuff, snus, and dissolvable tobacco products. Smokeless tobacco products typically contain over 3000 constituents. All smokeless tobacco products contain nicotine and are therefore highly addictive. Quitting smokeless tobacco use is as challenging as smoking cessation.

18-Methoxycoronaridine is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.

Nicotiana tabacum, or cultivated tobacco, is an annually grown herbaceous plant of the Nicotiana genus. The plant is tropical in origin, is commonly grown throughout the world, and is often found in cultivation. It grows to heights between 1 and 2 meters. Research is ongoing into its ancestry among wild Nicotiana species, but it is believed to be a hybrid of Nicotiana sylvestris, Nicotiana tomentosiformis, and possibly Nicotiana otophora. It is the most commonly grown of all plants in the genus Nicotiana, the plants' leaves commercially grown to be processed into tobacco.
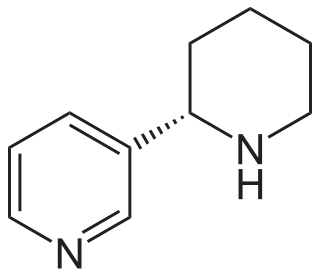
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco plant, as well as in the close relative of the common tobacco plant. It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.
Pituri, also known as mingkulpa, is a mixture of leaves and wood ash traditionally chewed as a stimulant by Aboriginal Australians widely across the continent. Leaves are gathered from any of several species of native tobacco (Nicotiana) or from at least one distinct population of the species Duboisia hopwoodii. Various species of Acacia, Grevillea and Eucalyptus are burned to produce the ash. The term "pituri" may also refer to the plants from which the leaves are gathered or from which the ash is made. Some authors use the term to refer only to the plant Duboisia hopwoodii and its leaves and any chewing mixture containing its leaves.

Lobeline is a pyridine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.

Cholinergic receptor, nicotinic, alpha 6, also known as nAChRα6, is a protein that in humans is encoded by the CHRNA6 gene. The CHRNA6 gene codes for the α6 nicotinic receptor subunit that is found in certain types of nicotinic acetylcholine receptors found primarily in the brain. Neural nicotinic acetylcholine receptors containing α6 subunits are expressed on dopamine-releasing neurons in the midbrain, and dopamine release following activation of these neurons is thought to be involved in the addictive properties of nicotine. Due to their selective localisation on dopaminergic neurons, α6-containing nACh receptors have also been suggested as a possible therapeutic target for the treatment of Parkinson's disease. In addition to nicotine, research in animals has implicated alpha-6-containing nAChRs in the abusive and addictive properties of ethanol, with mecamylamine demonstrating a potent ability to block these properties.

GBR-12935 is a piperazine derivative which is a potent and selective dopamine reuptake inhibitor. It was originally developed in its 3H radiolabelled form for the purpose of mapping the distribution of dopaminergic neurons in the brain by selective labelling of dopamine transporter proteins. This has led to potential clinical uses in the diagnosis of Parkinson's disease, although selective radioligands such as Ioflupane (¹²³I) are now available for this application. GBR-12935 is now widely used in animal research into Parkinson's disease and the dopamine pathways in the brain.

Duboisia hopwoodii is a shrub native to the arid interior region of Australia. Common names include pituri, pitchuri thornapple or pitcheri.

Anatabine (uh-nat-uh-been,-bin) is one of the minor alkaloids found in plants in the family Solanaceae, which includes the tobacco plant and tomato. Commercial tobacco plants typically produce alkaloids at levels between 2% and 4% of total dry weight, with nicotine accounting for about 90% of the total alkaloid content, and the related compounds anabatine, nornicotine, and anabasine making up nearly all the rest. These compounds are thought to be biologically active, and part of plants' natural defense system against insects.

Myosmine is an alkaloid found in tobacco and other plants. Chemically, it is closely related to nicotine. It inhibits aromatase seven fold more potently than nicotine. It also releases dopamine in adult but not adolescent rats.
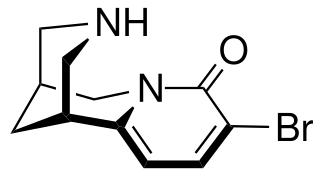
3-Bromocytisine is a derivative of the toxic alkaloid cytisine that acts as a highly potent agonist at neural nicotinic acetylcholine receptors, binding primarily to the α4β2 and α7 subtypes. 3-Bromocytisine is a full agonist at the α7 subtype while it is only a partial agonist at α4β2, but has an extremely strong binding affinity at α4β2 with 200-fold selectivity for α4β2 over α7. In animal studies 3-bromocytisine stimulates the release of dopamine and noradrenaline and increases locomotor activity.
The alpha-3 beta-2 nicotinic receptor, also known as the α3β2 receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β2 subunits.

Nicotryine is lesser known and minor tobacco alkaloid. It inhibits metabolism of nicotine through CYP2A6 enzyme inhibition. It also inhibits CYP2A13 which might play role in nicotine metabolism. Nicotyrine is formed by gradual oxidation of nicotine in e-liquids and causes delayed nicotine clearance and attenuated withdrawal symptoms.

The alpha-5 nicotinic acetylcholine receptor(α5 nAChR) also known as the α5 receptor is a type of ligand gated nicotinic acetylcholine receptor involved in pain regulation. One of the 5 transmembrane subunits of this receptor is the α5 subunit and is transcribed by the CHRNA5 gene. This receptor is commonly associated with nicotine addiction, immunotherapy, cancer, pain and attention.