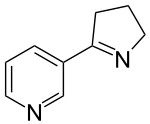
Nicotine is a naturally produced alkaloid in the nightshade family of plants and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits where it acts as a receptor antagonist.

Tobacco smoking is the practice of burning tobacco and ingesting the resulting smoke. The smoke may be inhaled, as is done with cigarettes, or simply released from the mouth, as is generally done with pipes and cigars. The practice is believed to have begun as early as 5000–3000 BC in Mesoamerica and South America. Tobacco was introduced to Eurasia in the late 17th century by European colonists, where it followed common trade routes. The practice encountered criticism from its first import into the Western world onwards but embedded itself in certain strata of a number of societies before becoming widespread upon the introduction of automated cigarette-rolling apparatus.
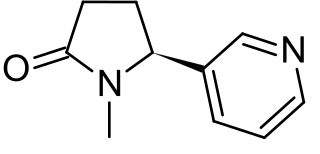
Cotinine is an alkaloid found in tobacco and is also the predominant metabolite of nicotine., typically used as a biomarker for exposure to tobacco smoke. Cotinine is currently being studied as a treatment for depression, post-traumatic stress disorder (PTSD), schizophrenia, Alzheimer's disease and Parkinson's disease. Cotinine was developed as an antidepressant as a fumaric acid salt, cotinine fumarate, to be sold under the brand name Scotine but it was never marketed.
The gateway drug effect is a comprehensive catchphrase for the often observed effect that the use of a psychoactive substance is coupled to an increased probability of the use of further substances. Possible causes are biological alterations in the brain due to the earlier substance exposure and similar attitudes of people who use different substances across different substances. In 2020, the National Institute on Drug Abuse released a study backing allegations that marijuana is a "gateway" to more dangerous substance use, though not for the majority of people who use substances. A literature review by the United States Department of Justice found no conclusive evidence that the link is causal.

Aromatase, also called estrogen synthetase or estrogen synthase, is an enzyme responsible for a key step in the biosynthesis of estrogens. It is CYP19A1, a member of the cytochrome P450 superfamily, which are monooxygenases that catalyze many reactions involved in steroidogenesis. In particular, aromatase is responsible for the aromatization of androgens into estrogens. The enzyme aromatase can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, and bone, as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer. It is an important factor in sexual development.

Nicotine poisoning describes the symptoms of the toxic effects of nicotine following ingestion, inhalation, or skin contact. Nicotine poisoning can potentially be deadly, though serious or fatal overdoses are rare. Historically, most cases of nicotine poisoning have been the result of use of nicotine as an insecticide. More recent cases of poisoning typically appear to be in the form of Green Tobacco Sickness, or due to unintended ingestion of tobacco or tobacco products or consumption of nicotine-containing plants.

18-Methoxycoronaridine is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.

Nicotiana tabacum, or cultivated tobacco, is an annually grown herbaceous plant of the genus Nicotiana. N. tabacum is the most commonly grown species in the genus Nicotiana, as the plant's leaves are commercially harvested to be processed into tobacco for human use. The plant is tropical in origin, is commonly grown throughout the world, and is often found in cultivation. It grows to heights between 1 and 2 meters. Research is ongoing into its ancestry among wild Nicotiana species, but it is believed to be a hybrid of Nicotiana sylvestris, N. tomentosiformis, and possibly N. otophora.
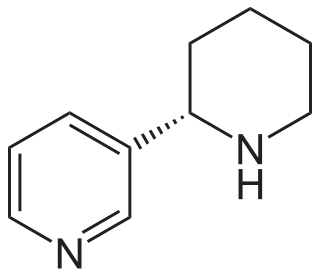
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco plant, as well as in the close relative of the common tobacco plant. It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.

Lobeline is a piperidine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.

An electronic cigarette is a device that simulates tobacco smoking. It consists of an atomizer, a power source such as a battery, and a container such as a cartridge or tank filled with liquid. Instead of smoke, the user inhales "vapor". As such, using an e-cigarette is often called "vaping". The atomizer is a heating element that vaporizes a liquid solution called e-liquid, which quickly cools into an aerosol of tiny droplets, vapor and air. E-cigarettes are activated by taking a puff or pressing a button. Some look like traditional cigarettes, and most kinds are reusable. The vapor mainly comprises propylene glycol and/or glycerin, usually with nicotine and flavoring. Its exact composition varies, and depends on several things including user behavior.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Flavored tobacco products — tobacco products with added flavorings — include types of cigarettes, cigarillos and cigars, hookahs and hookah tobacco, various types of smokeless tobacco, and more recently electronic cigarettes. Flavored tobacco products are especially popular with youth and have therefore become targets of regulation in several countries.

Ibogamine is an anti-convulsant, anti-addictive, CNS stimulant alkaloid found in Tabernanthe iboga and Crepe Jasmine. Basic research related to how addiction affects the brain has used this chemical.

BP-897 is a drug used in scientific research which acts as a potent selective dopamine D3 receptor partial agonist with an in vitro intrinsic activity of ~0.6 and ~70x greater affinity for D3 over D2 receptors and is suspected to have partial agonist or antagonist activity in vivo. It has mainly been used in the study of treatments for cocaine addiction. A study comparing BP-897 with the potent, antagonistic, and highly D3 selective SB-277,011-A found, "SB 277011-A (1–10 mg/kg) was able to block cue-induced reinstatement of nicotine-seeking, indicating that DRD3 selective antagonism may be an effective approach to prevent relapse for nicotine. In contrast, BP 897 did not block the cue-induced reinstatement of nicotine-seeking or nicotine-taking under the FR5 schedule."

Nornicotine is an alkaloid found in various plants including Nicotiana, the tobacco plant. It is chemically similar to nicotine, but does not contain a methyl group.
Iboga-type alkaloids are a set of monoterpene indole alkaloids comprising naturally-occurring compounds found in Tabernanthe and Tabernaemontana, as well as synthetic structural analogs. Naturally-occuring iboga-type alkaloids include ibogamine, ibogaine, tabernanthine, and other substituted ibogamines (see below). Many iboga-type alkaloids display biological activities such as cardiac toxicity and psychoactive effects, and some have been studied as potential treatments for drug addiction.

The usage of electronic cigarettes has risen rapidly since their introduction to the market in 2002. The global number of adult e-cigarettes users rose from about 7 million in 2011 to between 68 million and 82 million in 2021. Awareness and use of e-cigarettes greatly increased over the few years leading up to 2014, particularly among young people and women in some parts of the world. Since their introduction vaping has increased in the majority of high-income countries. E-cigarette use in the US and Europe is higher than in other countries, except for China which has the greatest number of e-cigarette users. Growth in the UK as of January 2018 had reportedly slowed since 2013. The growing frequency of e-cigarette use may be due to heavy promotion in youth-driven media channels, their low cost, and the belief that e-cigarettes are safer than traditional cigarettes, according to a 2016 review. E-cigarette use may also be increasing due to the consensus among several scientific organizations that e-cigarettes are safer compared to combustible tobacco products. E-cigarette use also appears to be increasing at the same time as a rapid decrease in cigarette use in many countries, suggesting that e-cigarettes may be displacing traditional cigarettes.
Exposure to nicotine, from conventional or electronic cigarettes during adolescence can impair the developing human brain. E-cigarette use is recognized as a substantial threat to adolescent behavioral health. The use of tobacco products, no matter what type, is almost always started and established during adolescence when the developing brain is most vulnerable to nicotine addiction. Young people's brains build synapses faster than adult brains. Because addiction is a form of learning, adolescents can get addicted more easily than adults. The nicotine in e-cigarettes can also prime the adolescent brain for addiction to other drugs such as cocaine. Exposure to nicotine and its great risk of developing an addiction, are areas of significant concern.

Nicotryine is lesser known and minor tobacco alkaloid. It inhibits metabolism of nicotine through CYP2A6 enzyme inhibition. It also inhibits CYP2A13 which might play role in nicotine metabolism. Nicotyrine is formed by gradual oxidation of nicotine in e-liquids and causes delayed nicotine clearance and attenuated withdrawal symptoms.