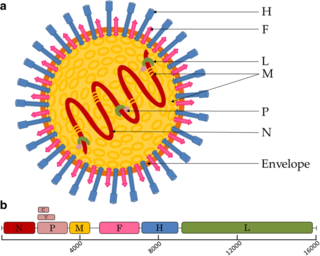
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.
Caulimoviridae is a family of viruses infecting plants. There are 94 species in this family, assigned to 11 genera. Viruses belonging to the family Caulimoviridae are termed double-stranded DNA (dsDNA) reverse-transcribing viruses i.e. viruses that contain a reverse transcription stage in their replication cycle. This family contains all plant viruses with a dsDNA genome that have a reverse transcribing phase in their lifecycle.

Potyviridae is a family of positive-strand RNA viruses that encompasses more than 30% of known plant viruses, many of which are of great agricultural significance. The family has 12 genera and 235 species, three of which are unassigned to a genus.

Viral replication is the formation of biological viruses during the infection process in the target host cells. Viruses must first get into the cell before viral replication can occur. Through the generation of abundant copies of its genome and packaging these copies, the virus continues infecting new hosts. Replication between viruses is greatly varied and depends on the type of genes involved in them. Most DNA viruses assemble in the nucleus while most RNA viruses develop solely in cytoplasm.
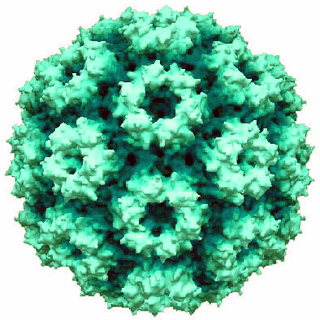
Cowpea chlorotic mottle virus, known by the abbreviation CCMV, is a virus that specifically infects the cowpea plant, or black-eyed pea. The leaves of infected plants develop yellow spots, hence the name "chlorotic". Similar to its "brother" virus, Cowpea mosaic virus (CPMV), CCMV is produced in high yield in plants. In the natural host, viral particles can be produced at 1–2 mg per gram of infected leaf tissue. Belonging to the bromovirus genus, cowpea chlorotic mottle virus (CCMV) is a small spherical plant virus. Other members of this genus include the brome mosaic virus (BMV) and the broad bean mottle virus (BBMV).

Alfalfa mosaic virus (AMV), also known as Lucerne mosaic virus or Potato calico virus, is a worldwide distributed phytopathogen that can lead to necrosis and yellow mosaics on a large variety of plant species, including commercially important crops. It is the only Alfamovirus of the family Bromoviridae. In 1931 Weimer J.L. was the first to report AMV in alfalfa. Transmission of the virus occurs mainly by some aphids, by seeds or by pollen to the seed.
Rice hoja blanca tenuivirus (RHBV), meaning "white leaf rice virus", is a plant virus in the family Phenuiviridae. RHBV causes Hoja blanca disease (HBD), which affects the leaves of the rice plant Oryza sativa, stunting the growth of the plant or killing it altogether. RHBV is carried by an insect vector, Tagosodes orizicolus, a type of planthopper. The virus is found in South America, Mexico, throughout Central America, the Caribbean region, and the southern United States. In South America, the disease is endemic to Colombia, Venezuela, Ecuador, Peru, Suriname, French Guiana and Guyana.

Cymbidium mosaic virus (CymMV) is a plant pathogenic virus of the family Alphaflexiviridae.
Strawberry crinkle cytorhabdovirus, commonly called Strawberry crinkle virus (SCV), is a negative sense single stranded RNA virus that threatens strawberry production worldwide. This virus reduces plant rigidity, runner production, fruit size, and production, while causing distortion and crinkling of the leaves. This virus was first described in 1932 in Oregon and California with commercial strawberry varieties, and later became an issue around the world, including North America, South America, Europe, South Africa, New Zealand, Australia, and Japan. Of the family Rhabdoviridae, it is a large family of viruses that affects plants, vertebrates, and invertebrates. Specifically, this virus infects strawberry plants of the genus Fragaria and is transmitted through two aphid vectors that feed on strawberries, Chaetosiphon fragaefolii and C. jacobi. When SCV is combined with other aphid-transmitted strawberry viruses, such as mottle, mild yellow-edge, vein banding, or pallidosis, the damage becomes even more deleterious. Economically, the only significant host of SCV is Fragaria ananassa.

Carlavirus, formerly known as the "Carnation latent virus group", is a genus of viruses in the order Tymovirales, in the family Betaflexiviridae. Plants serve as natural hosts. There are 53 species in this genus. Diseases associated with this genus include: mosaic and ringspot symptoms.
Cilevirus is a genus of viruses in the family Kitaviridae. Plants serve as natural hosts. There are two species: Citrus leprosis virus C and Citrus leprosis virus C2.
Dichorhavirus is a genus of negative sense, single-stranded RNA viruses of plants within the family Rhabdoviridae. Dichorhaviruses have segmented genomes and their short bacilliform virions are not enveloped. Dichorhaviruses are transmitted by mites.

Fig mosaic emaravirus (FMV) is a segmented, negative sense, single-stranded RNA virus that is determined to be the causal agent of fig mosaic disease (FMD) in fig plants, Ficus carica. It is a member of the genus Emaravirus and order Bunyavirales and is transmitted mainly by the eriophyid mite Aceria ficus. FMV can cause a range of symptoms varying in severity, including leaf chlorosis, deformity, and mosaic or discoloration patterns, as well as premature fruit drop.
Citrus leprosis(CL) is an economically important viral disease affecting citrus crops. This emerging disease is widely distributed in South and Central America, from Argentina to Mexico. The disease is associated with up to three different non-systemic viruses, which cause similar symptoms in the citrus hosts and are transmitted by the same vector, mites of the genus Brevipalpus; although they have vastly different genomes. Citrus leprosis virus nuclear type (CiLV-N) is found in the nuclei and cytoplasm of infected cells, while Citrus leprosis virus cytoplasmic type (CiLV-C) is found in the endoplasmic reticulum. In 2012, a new virus causing similar symptoms was found in Colombia and it was named Citrus leprosis virus cytoplasmic type 2 (CiLV-C2) due to its close similarity to CiLV-C. The cytoplasmic type viruses are the most prevalent and widely distributed of the three species.
Lily virus X (LVX) is a pathogenic ssRNA(+) plant virus of the family Alphaflexiviridae and the order Tymovirales.

Avian metaavulavirus 2, formerly Avian paramyxovirus 2, is a species of virus belonging to the family Paramyxoviridae and genus Metaavulavirus. The virus is a negative strand RNA virus containing a monopartite genome. Avian metaavulavirus 2 is one of nine species belonging to the genus Metaavulavirus. The most common serotype of Avulavirinae is serotype 1, the cause of Newcastle disease (ND). Avian metaavulavirus 2 has been known to cause disease, specifically mild respiratory infections in domestic poultry, including turkeys and chickens, and has many economic effects on egg production and poultry industries. The virus was first isolated from a strain in Yucaipa, California in 1956. Since then, other isolates of the virus have been isolated worldwide.

Botourmiaviridae is a family of positive-strand RNA viruses which infect plants and fungi. The family includes four genera: Ourmiavirus, Botoulivirus, Magoulivirus and Scleroulivirus. Members of genus Ourmiavirus infect plants and the other genera infect fungi. The member viruses have genomes which range from 2900 to 4800 nucleotides.











