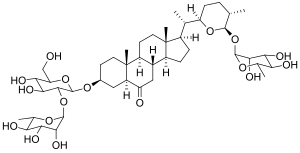
Stevia is a natural sweetener and sugar substitute derived from the leaves of the plant species Stevia rebaudiana, native to Brazil and Paraguay.

Fructose, or fruit sugar, is a simple ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into blood during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, berries, and most root vegetables.

A sugar substitute is a food additive that provides a sweet taste like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie (non-nutritive) or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar alcohols such as erythritol, xylitol, and sorbitol are derived from sugars. In 2017, sucralose was the most common sugar substitute used in the manufacture of foods and beverages; it had 30% of the global market, which was projected to be valued at $2.8 billion by 2021.
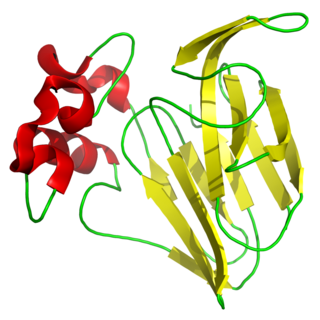
Thaumatin is a low-calorie sweetener and flavour modifier. The protein is often used primarily for its flavour-modifying properties and not exclusively as a sweetener.

Acesulfame potassium, also known as acesulfame K or Ace K, is a calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
Saponins, also referred to selectively as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, and the soapbark tree. They are used in soaps, medicinals, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages. Structurally, they are glycosides, sugars attached to another organic molecule, usually a steroid or triterpene, a steroid building block. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin, licorice flavoring; quillaia(alt. quillaja), a bark extract used in beverages; and squalene, a biological precursor to cholesterol that has been used as a vaccine adjuvant.

Monellin, a sweet protein, was discovered in 1969 in the fruit of the West African shrub known as serendipity berry ; it was first reported as a carbohydrate. The protein was named in 1972 after the Monell Chemical Senses Center in Philadelphia, U.S.A., where it was isolated and characterized.

Stevia rebaudiana is a plant species in the genus Stevia of the sunflower family (Asteraceae). It is commonly known as candyleaf, sweetleaf or sugarleaf.
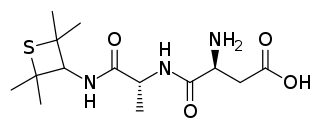
Alitame is an aspartic acid-containing dipeptide sweetener. It was developed by Pfizer in the early 1980s and currently marketed in some countries under the brand name Aclame. Most dipeptides are not sweet, but the unexpected discovery of aspartame in 1965 led to a search for similar compounds that shared its sweetness. Alitame is one such second-generation dipeptide sweetener. Neotame, developed by the owners of the NutraSweet brand, is another.

Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable, except when in excess. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketones, and sugar alcohols. Some are sweet at very low concentrations, allowing their use as non-caloric sugar substitutes. Such non-sugar sweeteners include saccharin and aspartame. Other compounds, such as miraculin, may alter perception of sweetness itself.

Gymnema sylvestre is a perennial woody vine native to tropical Asia, China, the Arabian Peninsula, Africa, and Australia. It has been used in Ayurvedic medicine. Common names include gymnema, Australian cowplant, and Periploca of the woods, and the Hindi term gurmar, which means "sugar destroyer".
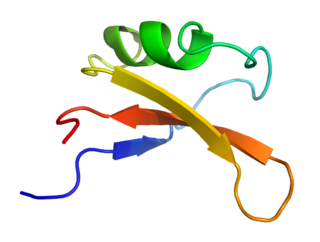
Brazzein is a sweet-tasting protein extracted from the West African fruit of the climbing plant Oubli. It was first isolated by the University of Wisconsin–Madison in 1994.

A mogroside is a glycoside of cucurbitane derivatives found in certain plants, such as the fruit of the gourd vine, luo han guo. Mogrosides are extracted from S. grosvenorii and used in the manufacture of sugar substitutes.

Steviol glycosides are the chemical compounds responsible for the sweet taste of the leaves of the South American plant Stevia rebaudiana (Asteraceae) and the main ingredients of many sweeteners marketed under the generic name stevia and several trade names. They also occur in the related species Stevia phlebophylla and in the plant Rubus chingii (Rosaceae).

Polypodium vulgare, the common polypody, is a fern of the family Polypodiaceae. Polypodium vulgare is an allotetraploid species, believed to have arisen by chromosome doubling of a sterile diploid hybrid between two ferns which are not known in Europe. The fern's proposed parents are the northern Asian and northern North American Polypodium sibiricum and western North American Polypodium glycyrrhiza. Biochemical data point to a species from eastern Asia as the second possible parent. The name is derived from poly (many) and pous, podos . Polypody has traditional uses in cooking for its aroma and sweet taste, and in herbal medicine as a purgative and vermifuge.

Thaumatococcus daniellii is a plant species from Africa, known for being the natural source of thaumatin, an intensely sweet protein which is of interest in the development of sweeteners. When the fleshy part of the fruit is eaten, this molecule binds to the tongue's taste buds, causing sour foods to taste sweet. It is a large, rhizomatous, flowering herb native to the rainforests of western Africa from Sierra Leone to Democratic Republic of the Congo. It is also an introduced species in Australia and Singapore.

Polypodium glycyrrhiza, commonly known as licorice fern, many-footed fern, and sweet root, is a summer deciduous fern native to western North America, where it is found in shaded, damp locations.

Rebaudioside A is a steviol glycoside from the leaves of Stevia rebaudiana that is 240 times sweeter than sugar. Rebaudioside A is the sweetest and most stable steviol glycoside, and is less bitter than stevioside. Stevia leaves contain 9.1% stevioside and 3.8% rebaudioside A.
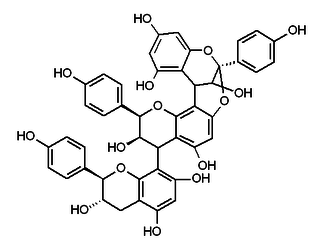
Selligueain A is an A type proanthocyanidin trimer of the propelargonidin type.