
Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. It is coded for by the gene APP and regulated by substrate presentation. APP is best known as the precursor molecule whose proteolysis generates amyloid beta (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients.

Secretases are enzymes that "snip" pieces off a longer protein that is embedded in the cell membrane.

Properdin is a protein that in humans is encoded by the CFP gene.
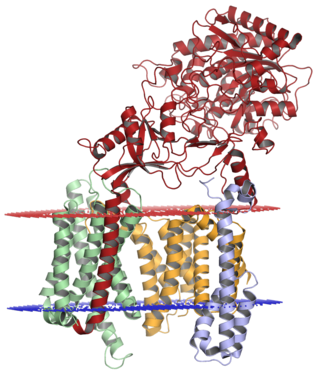
Gamma secretase is a multi-subunit protease complex, itself an integral membrane protein, that cleaves single-pass transmembrane proteins at residues within the transmembrane domain. Proteases of this type are known as intramembrane proteases. The most well-known substrate of gamma secretase is amyloid precursor protein, a large integral membrane protein that, when cleaved by both gamma and beta secretase, produces a short 37-43 amino acid peptide called amyloid beta whose abnormally folded fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Gamma secretase is also critical in the related processing of several other type I integral membrane proteins, such as Notch, ErbB4, E-cadherin, N-cadherin, ephrin-B2, or CD44.

Presenilins are a family of related multi-pass transmembrane proteins which constitute the catalytic subunits of the gamma-secretase intramembrane protease protein complex. They were first identified in screens for mutations causing early onset forms of familial Alzheimer's disease by Peter St George-Hyslop. Vertebrates have two presenilin genes, called PSEN1 that codes for presenilin 1 (PS-1) and PSEN2 that codes for presenilin 2 (PS-2). Both genes show conservation between species, with little difference between rat and human presenilins. The nematode worm C. elegans has two genes that resemble the presenilins and appear to be functionally similar, sel-12 and hop-1.
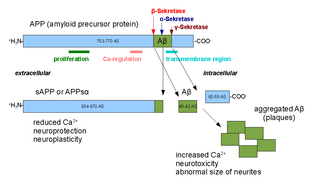
Alpha secretases are a family of proteolytic enzymes that cleave amyloid precursor protein (APP) in its transmembrane region. Specifically, alpha secretases cleave within the fragment that gives rise to the Alzheimer's disease-associated peptide amyloid beta when APP is instead processed by beta secretase and gamma secretase. The alpha-secretase pathway is the predominant APP processing pathway. Thus, alpha-secretase cleavage precludes amyloid beta formation and is considered to be part of the non-amyloidogenic pathway in APP processing. Alpha secretases are members of the ADAM family, which are expressed on the surfaces of cells and anchored in the cell membrane. Several such proteins, notably ADAM10, have been identified as possessing alpha-secretase activity. Upon cleavage by alpha secretases, APP releases its extracellular domain - a fragment known as APPsα - into the extracellular environment in a process known as ectodomain shedding.

Nicastrin, also known as NCSTN, is a protein that in humans is encoded by the NCSTN gene.
APH-1 is a protein gene product originally identified in the Notch signaling pathway in Caenorhabditis elegans as a regulator of the cell-surface localization of nicastrin. APH-1 homologs in other organisms, including humans, have since been identified as components of the gamma secretase complex along with the catalytic subunit presenilin and the regulatory subunits nicastrin and PEN-2. The gamma-secretase complex is a multimeric protease responsible for the intramembrane proteolysis of transmembrane proteins such as the Notch protein and amyloid precursor protein (APP). Gamma-secretase cleavage of APP is one of two proteolytic steps required to generate the peptide known as amyloid beta, whose misfolded form is implicated in the causation of Alzheimer's disease. All of the components of the gamma-secretase complex undergo extensive post-translational modification, especially proteolytic activation; APH-1 and PEN-2 are regarded as regulators of the maturation process of the catalytic component presenilin. APH-1 contains a conserved alpha helix interaction motif glycine-X-X-X-glycine (GXXXG) that is essential to both assembly of the gamma secretase complex and to the maturation of the components.

Presenilin-1(PS-1) is a presenilin protein that in humans is encoded by the PSEN1 gene. Presenilin-1 is one of the four core proteins in the gamma secretase complex, which is considered to play an important role in generation of amyloid beta (Aβ) from amyloid-beta precursor protein (APP). Accumulation of amyloid beta is associated with the onset of Alzheimer's disease.
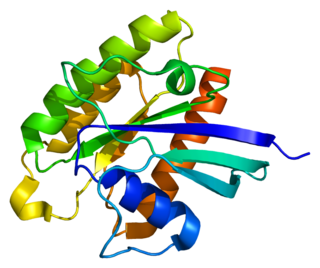
RHEB also known as Ras homolog enriched in brain (RHEB) is a GTP-binding protein that is ubiquitously expressed in humans and other mammals. The protein is largely involved in the mTOR pathway and the regulation of the cell cycle.

Presenilin-2 is a protein that is encoded by the PSEN2 gene.

Delta-like protein 1 is a protein that in humans is encoded by the DLL1 gene.

5'-AMP-activated protein kinase subunit gamma-1 is an enzyme that in humans is encoded by the PRKAG1 gene.

Transmembrane emp24 domain-containing protein 10 is a protein that in humans is encoded by the TMED10 gene.

Jagged-2 is a protein that in humans is encoded by the JAG2 gene.

Ubiquilin-2 is a protein that in humans is encoded by the UBQLN2 gene.

Signal peptide peptidase-like 2B, also known as SPPL2B, is a human gene.

Sodium channel subunit beta-2 is a protein that in humans is encoded by the SCN2B gene.

Transmembrane emp24 domain-containing protein 2 is a protein that in humans is encoded by the TMED2 gene.

Protein pigeon homolog also known as gamma-secretase activating protein (GSAP) is a protein that in humans is encoded by the PION gene.



















