
Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled.

Aciclovir (ACV), also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected.

Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.
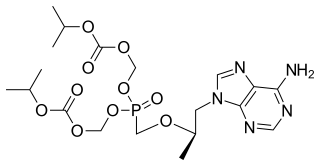
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.

Cidofovir, brand name Vistide, is a topical or injectable antiviral medication primarily used as a treatment for cytomegalovirus (CMV) retinitis in people with AIDS.

Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates.

Brivudine is an antiviral drug used in the treatment of herpes zoster ("shingles"). Like other antivirals, it acts by inhibiting replication of the target virus.

Ateviridine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. It was invented by Professor Chris McGuigan in the early 1990s. They form a critical part of the anti-viral drugs: sofosbuvir, tenofovir alafenamide and remdesivir.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.

Rabacfosadine, sold under the brand name Tanovea-CA1, is a guanine nucleotide analog used for the treatment of lymphoma in dogs. The drug was granted conditional approval by the U.S. Food and Drug Administration under application number 141-475 for use in treating canine lymphoma in December 2016 pending a full demonstration of effectiveness, and became the first drug to receive full approval for the treatment of canine lymphoma in July 2021.
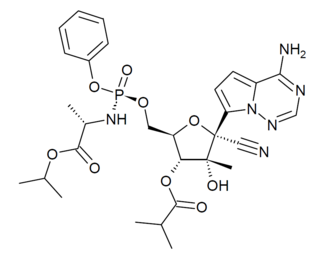
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
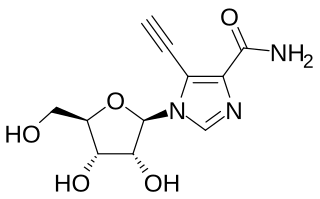
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.
Katherine Seley-Radtke is an American medicinal chemist who specializes in the discovery and design of novel nucleoside or nucleotide based enzyme inhibitors that may be used to treat infections or cancer. She has authored over 90 peer-reviewed publications,is an inventor of five issued US patents, and is a Professor in the Department of Chemistry & Biochemistry at the University of Maryland, Baltimore County. Her international impact includes scientific collaborations, policy advising and diplomatic appointments in biosecurity efforts.
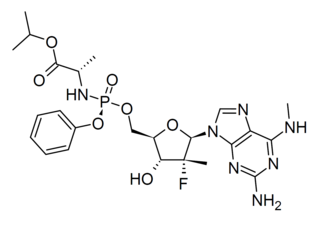
Bemnifosbuvir is an antiviral drug invented by Atea Pharmaceuticals and licensed to Roche for clinical development, a novel nucleotide analog prodrug originally developed for the treatment of hepatitis C. AT-527 is the orally bioavailable hemisulfate salt of AT-511, which is metabolised in several steps to the active nucleotide triphosphate AT-9010, acting as an RNA polymerase inhibitor and thereby interfering with viral replication. AT-527 has been researched for the treatment of coronavirus diseases such as that produced by SARS-CoV-2. It showed good results in early clinical trials but had inconsistent results at later stages, so the planned Phase 3 trials are being redesigned and results are not expected until late 2022.

Obeldesivir is an isobutyl ester prodrug of GS-441524 made by Gilead Sciences that is currently in Phase III trials for the outpatient treatment of COVID-19 in high risk patients. The purpose of the isopropyl ester modification on obeldesivir is to improve the oral bioavailability of the parent nucleoside, GS-441524. Obeldesivir is hydrolyzed to its parent nucleoside, GS-441524, which is in turn converted to remdesivir-triphosphate (GS-443902) by a nucleoside kinase, adenylate kinase and nucleotide diphosphate kinase.
















