
X-ray fluorescence (XRF) is the emission of characteristic "secondary" X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings.

A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light. Because light is an electromagnetic wave, other forms of electromagnetic radiation such as X-rays, microwaves, and radio waves exhibit similar properties.

In the field of optics, transparency is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale, the photons can be said to follow Snell's law. Translucency allows light to pass through but does not necessarily follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposite property of translucency is opacity. Other categories of visual appearance, related to the perception of regular or diffuse reflection and transmission of light, have been organized under the concept of cesia in an order system with three variables, including transparency, translucency and opacity among the involved aspects.

A dye laser is a laser that uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths, often spanning 50 to 100 nanometers or more. The wide bandwidth makes them particularly suitable for tunable lasers and pulsed lasers. The dye rhodamine 6G, for example, can be tuned from 635 nm (orangish-red) to 560 nm (greenish-yellow), and produce pulses as short as 16 femtoseconds. Moreover, the dye can be replaced by another type in order to generate an even broader range of wavelengths with the same laser, from the near-infrared to the near-ultraviolet, although this usually requires replacing other optical components in the laser as well, such as dielectric mirrors or pump lasers.

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.
Liquid crystal on silicon is a miniaturized reflective active-matrix liquid-crystal display or "microdisplay" using a liquid crystal layer on top of a silicon backplane. It is also known as a spatial light modulator. LCoS initially was developed for projection televisions, but has since found additional uses in wavelength selective switching, structured illumination, near-eye displays and optical pulse shaping.
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator, and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection.

Gamma-ray spectroscopy is the qualitative study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acquire a quantitative spectrum measurement.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F), equivalent to a wavelength of 459 nm. ZnSe occurs as the rare mineral stilleite, named after Hans Stille.

Bismuth germanium oxide or bismuth germanate is an inorganic chemical compound of bismuth, germanium and oxygen. Most commonly the term refers to the compound with chemical formula Bi4Ge3O12 (BGO), with the cubic evlitine crystal structure, used as a scintillator. (The term may also refer to a different compound with formula Bi12GeO20, an electro-optical material with sillenite structure, and Bi2Ge3O9.)
Cadmium tungstate (CdWO4 or CWO), the cadmium salt of tungstic acid, is a dense, chemically inert solid which is used as a scintillation crystal to detect gamma rays. It has density of 7.9 g/cm3 and melting point of 1325 °C. It is toxic if inhaled or swallowed. Its crystals are transparent, colorless, with slight yellow tint. It is odorless. Its CAS number is 7790-85-4. It is not hygroscopic.

DEAP is a direct dark matter search experiment which uses liquid argon as a target material. DEAP utilizes background discrimination based on the characteristic scintillation pulse-shape of argon. A first-generation detector (DEAP-1) with a 7 kg target mass was operated at Queen's University to test the performance of pulse-shape discrimination at low recoil energies in liquid argon. DEAP-1 was then moved to SNOLAB, 2 km below Earth's surface, in October 2007 and collected data into 2011.
Gadolinium oxysulfide (Gd2O2S), also called gadolinium sulfoxylate, GOS or Gadox, is an inorganic compound, a mixed oxide-sulfide of gadolinium.
The optical properties of all liquid and solid materials change as a function of the wavelength of light used to measure them. This change as a function of wavelength is called the dispersion of the optical properties. The graph created by plotting the optical property of interest by the wavelength at which it is measured is called a dispersion curve.
Lanthanum(III) bromide (LaBr3) is an inorganic halide salt of lanthanum. When pure, it is a colorless white powder. The single crystals of LaBr3 are hexagonal crystals with melting point of 783 °C. It is highly hygroscopic and water-soluble. There are several hydrates, La3Br·x H2O, of the salt also known. It is often used as a source of lanthanum in chemical synthesis and as a scintillation material in certain applications.
In phosphors and scintillators, the activator is the element added as dopant to the crystal of the material to create desired type of nonhomogeneities.
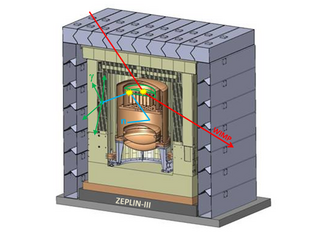
The ZEPLIN-III dark matter experiment attempted to detect galactic WIMPs using a 12 kg liquid xenon target. It operated from 2006 to 2011 at the Boulby Underground Laboratory in Loftus, North Yorkshire. This was the last in a series of xenon-based experiments in the ZEPLIN programme pursued originally by the UK Dark Matter Collaboration (UKDMC). The ZEPLIN-III project was led by Imperial College London and also included the Rutherford Appleton Laboratory and the University of Edinburgh in the UK, as well as LIP-Coimbra in Portugal and ITEP-Moscow in Russia. It ruled out cross-sections for elastic scattering of WIMPs off nucleons above 3.9 × 10−8 pb from the two science runs conducted at Boulby.

2,5-Diphenyloxazole (PPO) is an organic scintillator. It is used as a wavelength shifter, which means that it converts shorter wavelength light to longer wavelength light. Its output spectrum peaks at 385 nm, which is in the range of UV light.












