
Zingiberaceae or the ginger family is a family of flowering plants made up of about 50 genera with a total of about 1600 known species of aromatic perennial herbs with creeping horizontal or tuberous rhizomes distributed throughout tropical Africa, Asia, and the Americas. Many of the family's species are important ornamental, spice, or medicinal plants. Ornamental genera include the shell gingers (Alpinia), Siam or summer tulip, Globba, ginger lily (Hedychium), Kaempferia, torch-ginger Etlingera elatior, Renealmia, and ginger (Zingiber). Spices include ginger (Zingiber), galangal or Thai ginger, melegueta pepper, myoga, korarima, turmeric (Curcuma), and cardamom.

Grains of paradise is a species in the ginger family, Zingiberaceae, and closely related to cardamom. Its seeds are used as a spice ; it imparts a pungent, black-pepper-like flavor with hints of citrus. It is also known as melegueta pepper, Guinea grains, ossame, or fom wisa, and is sometimes confused with alligator pepper. The terms African pepper and Guinea pepper have also been used, but are ambiguous as they can apply to other spices such as grains of Selim.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels (angiogenesis). Some angiogenesis inhibitors are endogenous and a normal part of the body's control and others are obtained exogenously through pharmaceutical drugs or diet.

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates heat receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.

Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Caryophyllene, more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
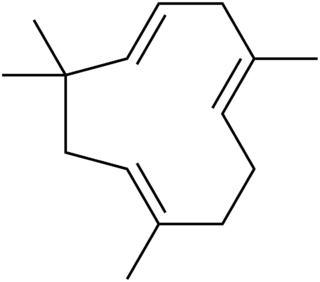
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.

Seliciclib is an experimental drug candidate in the family of pharmacological cyclin-dependent kinase (CDK) inhibitors that preferentially inhibit multiple enzyme targets including CDK2, CDK7 and CDK9, which alter the growth phase or state within the cell cycle of treated cells. Seliciclib is being developed by Cyclacel.This is a phase II, dose ranging, multicenter, randomized, double-blind, placebo-controlled study.

Ursolic acid, is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme.

Kaempferia galanga, commonly known as kencur, aromatic ginger, sand ginger, cutcherry, is a monocotyledonous plant in the ginger family, and one of four plants called galangal. It is found primarily in open areas in Indonesia, southern China, Taiwan, Cambodia, and India, but is also widely cultivated throughout Southeast Asia.

Nobiletin is a flavonoid isolated from citrus peels. It is an O-methylated flavone that has the activity to rescue bulbectomy-induced memory impairment.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

Aframomum corrorima is a species of flowering plant in the ginger family, Zingiberaceae. It is an herbaceous perennial that produces leafy stems 1–2 meters tall from rhizomatous roots. The alternately-arranged leaves are dark green, 10–30 cm long and 2.5–6 cm across, elliptical to oblong in shape. Pink flowers are borne near the ground and give way to red, fleshy fruits containing shiny brown seeds, which are typically 3–5 mm in diameter.
1,7-Bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one is a natural product, a curcuminoid antioxidant found in turmeric and torch ginger.

Dicycloplatin is a chemotherapy medication used to treat a number of cancers which includes the non-small-cell lung carcinoma and prostate cancer.

The history of spices reach back thousands of years, dating back to the 8th century B.C. Spices are widely known to be developed and discovered in Asian civilizations. Spices have been used in a variety of antique developments for their unique qualities. There were a variety of spices that were used for common purposes across the ancient world. Different spices hold a value that can create a variety of products designed to enhance or suppress certain taste and/or sensations. Spices were also associated with certain rituals to perpetuate a superstition or fulfill a religious obligation, among other things.

C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.

Securinine is an alkaloid found in Securinega suffruticosa and Phyllanthus niruri.

Aframomum daniellii, also known as African cardamom, is a species of flowering plant in the ginger family, Zingiberaceae. It was first described by Joseph Dalton Hooker, and got its current name from Karl Moritz Schumann.


















