The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Arsenopyrite is an iron arsenic sulfide (FeAsS). It is a hard metallic, opaque, steel grey to silver white mineral with a relatively high specific gravity of 6.1. When dissolved in nitric acid, it releases elemental sulfur. When arsenopyrite is heated, it produces sulfur and arsenic vapor. With 46% arsenic content, arsenopyrite, along with orpiment, is a principal ore of arsenic. When deposits of arsenopyrite become exposed to the atmosphere, the mineral slowly converts into iron arsenates. Arsenopyrite is generally an acid-consuming sulfide mineral, unlike iron pyrite which can lead to acid mine drainage.

A pnictogen is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc).

Vanadinite is a mineral belonging to the apatite group of phosphates, with the chemical formula Pb5(VO4)3Cl. It is one of the main industrial ores of the metal vanadium and a minor source of lead. A dense, brittle mineral, it is usually found in the form of red hexagonal crystals. It is an uncommon mineral, formed by the oxidation of lead ore deposits such as galena. First discovered in 1801 in Mexico, vanadinite deposits have since been unearthed in South America, Europe, Africa, and North America.

Bornite, also known as peacock ore, is a sulfide mineral with chemical composition Cu5FeS4 that crystallizes in the orthorhombic system (pseudo-cubic).

Stibnite, sometimes called antimonite, is a sulfide mineral with the formula Sb2S3. This soft grey material crystallizes in an orthorhombic space group. It is the most important source for the metalloid antimony. The name is derived from the Greek στίβι stibi through the Latin stibium as the former name for the mineral and the element antimony.

Nickeline or niccolite is a mineral consisting primarily of nickel arsenide (NiAs). The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly.

Realgar, also known as "ruby sulphur" or "ruby of arsenic", is an arsenic sulfide mineral with the chemical formula α-As4S4. It is a soft, sectile mineral occurring in monoclinic crystals, or in granular, compact, or powdery form, often in association with the related mineral, orpiment. It is orange-red in color, melts at 320 °C, and burns with a bluish flame releasing fumes of arsenic and sulfur. Realgar is soft with a Mohs hardness of 1.5 to 2 and has a specific gravity of 3.5. Its streak is orange colored. It is trimorphous with pararealgar and bonazziite. Its name comes from the Arabic rahj al-ġār, via Medieval Latin, and its earliest record in English is in the 1390s.

Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sn with n ≤ 10. Two are of commercial significance, phosphorus pentasulfide, which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide, used in the production of "strike anywhere matches".

Phosphorus sesquisulfide is the inorganic compound with the formula P4S3. It was developed by Henri Sevene and Emile David Cahen in 1898 as part of their invention of friction matches that did not pose the health hazards of white phosphorus. This yellow solid is one of two commercially produced phosphorus sulfides. It is a component of "strike anywhere" matches.

Lorándite is a thallium arsenic sulfosalt with the chemical formula: TlAsS2. Though rare, it is the most common thallium-bearing mineral. Lorandite occurs in low-temperature hydrothermal associations and in gold and mercury ore deposits. Associated minerals include stibnite, realgar, orpiment, cinnabar, vrbaite, greigite, marcasite, pyrite, tetrahedrite, antimonian sphalerite, arsenic and barite.

Djurleite is a copper sulfide mineral of secondary origin with formula Cu31S16 that crystallizes with monoclinic-prismatic symmetry. It is typically massive in form, but does at times develop thin tabular to prismatic crystals. It occurs with other supergene minerals such as chalcocite, covellite and digenite in the enriched zone of copper orebodies. It is a member of the chalcocite group, and very similar to chalcocite, Cu2S, in its composition and properties, but the two minerals can be distinguished from each other by x-ray powder diffraction. Intergrowths and transformations between djurleite, digenite and chalcocite are common. Many of the reported associations of digenite and djurleite, however, identified by powder diffraction, could be anilite and djurleite, as anilite transforms to digenite during grinding.

Penroseite is a rare selenide mineral with formula (Ni,Co,Cu)Se2. It has a gray-steel color and black streak with a hardness of 3. It is an isometric mineral, 2/m3. Penroseite was first discovered in 1925 in a Bolivian rhyolite. It was named for Richard Penrose (1863–1931), an economic geologist.
Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores.

Huttonite is a thorium nesosilicate mineral with the chemical formula ThSiO4 and which crystallizes in the monoclinic system. It is dimorphous with tetragonal thorite, and isostructual with monazite. An uncommon mineral, huttonite forms transparent or translucent cream–colored crystals. It was first identified in samples of beach sands from the West Coast region of New Zealand by the mineralogist Colin Osborne Hutton (1910–1971). Owing to its rarity, huttonite is not an industrially useful mineral.

Alacránite (As8S9) is an arsenic sulfide mineral first discovered in the Uzon caldera, Kamchatka, Russia. It was named for its occurrence in the Alacrán silver/arsenic/antimony mine. Pampa Larga, Chile. It is generally more rare than realgar and orpiment. Its origin is hydrothermal. It occurs as subhedral to euhedral tabular orange to pale gray crystals that are transparent to translucent. It has a yellow-orange streak with a hardness of 1.5. It crystallizes in the monoclinic crystal system. It occurs with realgar and uzonite as flattened and prismatic grains up to 0.5 mm across.

Bergenite is a rare uranyl phosphate of the more specific phosphuranylite group. The phosphuranylite-type sheet in bergenite is a new isomer of the group, with the uranyl phosphate tetrahedra varying in an up-up-down, same-same-opposite (uuduudSSOSSO) orientation. All bergenite samples have been found in old mine dump sites. Uranyl minerals are a large constituent of uranium deposits.

Getchellite is a rare sulfide of arsenic and antimony, AsSbS3, that was discovered by B. G. Weissberg of the New Zealand Department of Scientific and Industrial Research in 1963, and approved as a new species by the International Mineralogical Association in 1965. Many metal sulfides are grey to black, but a few are brightly colored. Orpiment is yellow to brownish gold, cinnabar is deep red and getchellite is a bright orange red.

Segnitite is a lead iron(III) arsenate mineral. Segnitite was first found in the Broken Hill ore deposit in Broken Hill, New South Wales, Australia. In 1991, segnitite was approved as a new mineral. Segnitite has since been found worldwide near similar locality types where rocks are rich in zinc and lead especially. it was named for Australian mineralogist, gemologist and petrologist Edgar Ralph Segnit. The mineral was named after E. R. Segnit due to his contributions to Australian mineralogy.

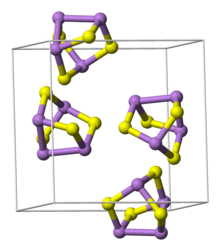
Compounds of arsenic resemble in some respects those of phosphorus which occupies the same group (column) of the periodic table. The most common oxidation states for arsenic are: −3 in the arsenides, which are alloy-like intermetallic compounds, +3 in the arsenites, and +5 in the arsenates and most organoarsenic compounds. Arsenic also bonds readily to itself as seen in the square As3−
4 ions in the mineral skutterudite. In the +3 oxidation state, arsenic is typically pyramidal owing to the influence of the lone pair of electrons.