
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Datura is a genus of nine species of highly poisonous, vespertine-flowering plants belonging to the nightshade family (Solanaceae). They are commonly known as thornapples or jimsonweeds, but are also known as devil's trumpets. Other English common names include moonflower, devil's weed, and hell's bells. All species of Datura are extremely poisonous and potentially psychoactive, especially their seeds and flowers, which can cause respiratory depression, arrhythmias, fever, delirium, hallucinations, anticholinergic syndrome, psychosis, and death if taken internally.
Yohimbine, also known as quebrachine, is an indole alkaloid derived from the bark of the African tree Pausinystalia johimbe; also from the bark of the unrelated South American tree Aspidosperma quebracho-blanco. Yohimbine is an α2-adrenergic receptor antagonist, and has been used in a variety of research projects. It is a veterinary drug used to reverse sedation in dogs and deer.

Curare is a common name for various alkaloid arrow poisons originating from plant extracts. Used as a paralyzing agent by indigenous peoples in Central and South America for hunting and for therapeutic purposes, curare only becomes active when it contaminates a wound or is introduced directly to the bloodstream; it is not active when ingested orally. These poisons cause weakness of the skeletal muscles and, when administered in a sufficient dose, eventual death by asphyxiation due to paralysis of the diaphragm. Curare is prepared by boiling the bark of one of the dozens of plant sources, leaving a dark, heavy paste that can be applied to arrow or dart heads. In medicine, curare has been used as a treatment for tetanus and strychnine poisoning and as a paralyzing agent for surgical procedures.
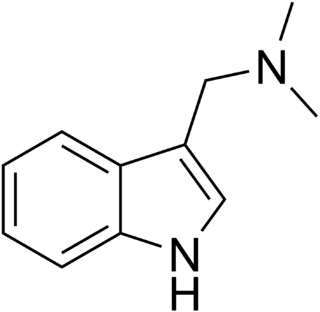
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.

Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which it was named.

Gelsemine (C20H22N2O2) is an indole alkaloid isolated from flowering plants of the genus Gelsemium, a plant native to the subtropical and tropical Americas, and southeast Asia, and is a highly toxic compound that acts as a paralytic, exposure to which can result in death. It has generally potent activity as an agonist of the mammalian glycine receptor, the activation of which leads to an inhibitory postsynaptic potential in neurons following chloride ion influx, and systemically, to muscle relaxation of varying intensity and deleterious effect. Despite its danger and toxicity, recent pharmacological research has suggested that the biological activities of this compound may offer opportunities for developing treatments related to xenobiotic or diet-induced oxidative stress, and of anxiety and other conditions, with ongoing research including attempts to identify safer derivatives and analogs to make use of gelsemine's beneficial effects.

Picralima is a plant genus in the family Apocynaceae, first described as a genus in 1896. It contains only one known species, Picralima nitida, native to tropical Africa.

Rugulovasines are bio-active alkaloids made by Penicillium. Rugulovasine A and B bind strongly to the 5-HT1A, 5-HT2A, and 5-HT2C receptors, but lack meaningful binding affinity towards the α1 adrenergic and dopamine receptors. Little is known about the in vivo activity of Rugulovasine A and B, although they have hypotensive effects in cats.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium cluniae is a fungus species of the genus of Penicillium which produces the antinematodal and antiparasitic agents paraherquamide B, paraherquamide C, paraherquamide D, paraherquamide E, paraherquamide F, paraherquamide G, paraherquamide H
Penicillium decaturense is a species of the genus of Penicillium which was isolated from a fungus in North America. Penicillium decaturense produces citrinin, 15-Deoxyoxalicine B, decaturins A and decaturins A
Penicillium lapatayae is an anamorph species of the genus of Penicillium which was isolated from Tierra del Fuego in Chile. Penicillium lapatayae produces (-)-lapatin B
Penicillium paneum is a species of fungus in the genus Penicillium which can spoil cereal grains. Penicillium paneum produces 1-Octen-3-ol and penipanoid A, penipanoid B, penipanoid C, patulin and roquefortine C
Penicillium rubrum is a species of fungus in the genus Penicillium which produces kojic acid, mitorubrin, mitorubrinol, rubratoxin A, rubratoxin B rubralactone, rubramin and occurs in grain corn and soybeans. Penicillium rubrum is similar to the species Penicillium chrysogenum.
Penicillium thymicola is a halotolerant species of fungus in the genus Penicillium which produces okaramine A, daldinin D, alantrypinone, seranttrypinone, fumiquinazoline F and fumiquinazoline G.
Penicillium vinaceum is an anamorph species of fungus in the genus Penicillium which produces penicillivinacine, vinaxanthone and citrmycetin.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.











