
Griseofulvin is an antifungal medication used to treat a number of types of dermatophytoses (ringworm). This includes fungal infections of the nails and scalp, as well as the skin when antifungal creams have not worked. It is taken by mouth.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.

Penicillium roqueforti is a common saprotrophic fungus in the genus Penicillium. Widespread in nature, it can be isolated from soil, decaying organic matter, and plants.

Dimethyl telluride is an organotelluride compound, formula (CH3)2Te, also known by the abbreviation DMTe.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.
Penicillium griseofulvum is a species of the genus of Penicillium which produces patulin, penifulvin A, cyclopiazonic acid, roquefortine C, shikimic acid, griseofulvin, and 6-Methylsalicylic acid. Penicillium griseofulvum occurs on cereals and nuts.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.
Leucogenenol is a blood cell stimulating secondary metabolite isolated from the mold Penicillium gilmanii. Its chemical structure was reported; however, later studies determined that the original structure is incorrect and the true chemical structure of leucogenenol remains unknown.
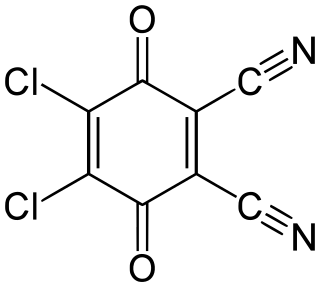
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid.

Secalonic acids are a group of xanthone derivatives closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses. From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified. Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium citrinum is an anamorph, mesophilic fungus species of the genus of Penicillium which produces tanzawaic acid A-D, ACC, Mevastatin, Quinocitrinine A, Quinocitrinine B, and nephrotoxic citrinin. Penicillium citrinum is often found on moldy citrus fruits and occasionally it occurs in tropical spices and cereals. This Penicillium species also causes mortality for the mosquito Culex quinquefasciatus. Because of its mesophilic character, Penicillium citrinum occurs worldwide. The first statin (Mevastatin) was 1970 isolated from this species.
Penicillium diversum is an anamorph species of the genus of Penicillium which produces austinol, isoaustin and diversonol.
Penicillium islandicum is an anamorph species of the genus of Penicillium which produces luteoskyrin, simatoxin, cyclochlorotine, rugulosin, islanditoxin and chitosanase.
Penicillium multicolor is an anamorph species of the genus Penicillium which produces alpha-L-fucosidase, tilactase, sclerotiorin, 8-O-Methylsclerotiorinamine, multicolosic acid and isochromophilones.
Penicillium paxilli is an anamorph, saprophytic species of the genus Penicillium which produces paxilline, paxisterol, penicillone, pyrenocine A, paspaline B and verruculogene. Penicillium paxilli is used as a model to study the biochemistry of the indol-diterepene biosynthesis

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.
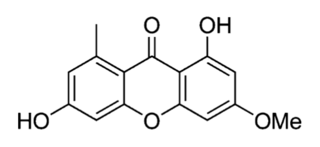
Griseoxanthone C is an organic compound in the structural class of chemicals known as xanthones. Its chemical formula is 1,6-dihydroxy-3-methoxy-8-methylxanthen-9-one, and its molecular formula is C15H12O5. It is found in a plant and some fungi, including a lichen.










