
Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib. This residue strongly induces formation of alpha-helical structure. The peptide sequence is

Komagataella is a methylotrophic yeast within the order Saccharomycetales. It was found in the 1960s as Pichia pastoris, with its feature of using methanol as a source of carbon and energy. In 1995, P. pastoris was reassigned into the sole representative of genus Komagataella, becoming Komagataella pastoris. Later studies have further distinguished new species in this genus, resulting in a total of 7 recognized species. It is not uncommon to see the old name still in use in the contect of protein production, as of 2023; in less formal use, the yeast may confusingly be referred to as pichia.
HNF4 is a nuclear receptor protein mostly expressed in the liver, gut, kidney, and pancreatic beta cells that is critical for liver development. In humans, there are two paralogs of HNF4, HNF4α and HNF4γ,encoded by two separate genes HNF4A and HNF4G respectively.

The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of E. coli. In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety.

T7 RNA Polymerase is an RNA polymerase from the T7 bacteriophage that catalyzes the formation of RNA from DNA in the 5'→ 3' direction.

6-Phosphogluconate dehydrogenase (6PGD) is an enzyme in the pentose phosphate pathway. It forms ribulose 5-phosphate from 6-phosphogluconate:
Thomas Alwyn Jones (born 30 August 1947) is a Welsh biophysicist and a professor at the Uppsala University in Sweden.

Collagen alpha-1(XVIII) chain is a protein that in humans is encoded by the COL18A1 gene.

Peroxiredoxin-2 is a protein that in humans is encoded by the PRDX2 gene.

NAD-dependent malic enzyme, mitochondrial is a protein that in humans is encoded by the ME2 gene. This gene encodes a mitochondrial NAD-dependent malic enzyme, a homotetrameric protein, that catalyzes the oxidative decarboxylation of malate to pyruvate. It had previously been weakly linked to a syndrome known as Friedreich ataxia that has since been shown to be the result of mutation in a completely different gene.
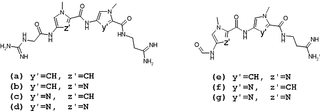
Lexitropsins are members of a family of semi-synthetic DNA-binding ligands. They are structural analogs of the natural antibiotics netropsin and distamycin. Antibiotics of this group can bind in the minor groove of DNA with different sequence-selectivity. Lexitropsins form a complexes with DNA with stoichiometry 1:1 and 2:1. Based on the 2:1 complexes were obtained ligands with high sequence-selectivity.

In molecular biology, glycoside hydrolase family 49 is a family of glycoside hydrolases.
Gerard Jacob Kleywegt is a Dutch X-ray crystallographer and the former team leader of the Protein Data Bank in Europe at the EBI; a member of the Worldwide Protein Data Bank.

Tetrahydrocannabinolic acid (THCA) synthase is an enzyme responsible for catalyzing the formation of THCA from cannabigerolic acid (CBGA). THCA is the direct precursor of tetrahydrocannabinol (THC), the principal psychoactive component of cannabis, which is produced from various strains of Cannabis sativa. Therefore, THCA synthase is considered to be a key enzyme controlling cannabis psychoactivity. Polymorphisms of THCA synthase result in varying levels of THC in Cannabis plants, resulting in "drug-type" and "fiber-type" C. sativa varieties.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Chitin-glucan complex (CGC) is a copolymer (polysaccharide) that makes up fungal cell walls, consisting of covalently-bonded chitin and branched 1,3/1,6-ß-D-glucan. CGCs are alkaline-insoluble. Different species of fungi have different structural compositions of chitin and β-glucan making up the CGCs in their cell walls. Soil composition and other environmental factors can also affect the ratio of chitin to β-glucan found in the CGC. Fungal cell walls may also contain chitosan-glucan complexes, which are similar copolymers but have chitosan instead of chitin. Chitin and chitosan are closely related molecules: greater than 40% of the polymer chain of chitin is made of acetylated glucosamine units, whereas greater than 60% of chitosan is made of deacetylated glucosamine units.
Penicillium chermesinum is an anamorph fungus species of the genus of Penicillium which was isolated from soil from Nova Scotia in Canada.Penicillium chermesinum produces plastatin, luteosporin, xanthomegnin, azaphilones, p-terphenyls and costaclavine.
Penicillium citrinum is an anamorph, mesophilic fungus species of the genus of Penicillium which produces tanzawaic acid A-D, ACC, Mevastatin, Quinocitrinine A, Quinocitrinine B, and nephrotoxic citrinin. Penicillium citrinum is often found on moldy citrus fruits and occasionally it occurs in tropical spices and cereals. This Penicillium species also causes mortality for the mosquito Culex quinquefasciatus. Because of its mesophilic character, Penicillium citrinum occurs worldwide. The first statin (Mevastatin) was 1970 isolated from this species.
Micromonospora viridifaciens is an endophytic actinomycete.

β-propeller phytases (BPPs) are a group of enzymes (i.e. protein superfamily) with a round beta-propeller structure. BPPs are phytases, which means that they are able to remove (hydrolyze) phosphate groups from phytic acid and its phytate salts. Hydrolysis happens stepwise and usually ends in myo-inositol triphosphate product which has three phosphate groups still bound to it. The actual substrate of BPPs is calcium phytate and in order to hydrolyze it, BPPs must have Ca2+ ions bound to themselves. BPPs are the most widely found phytase superfamily in the environment and they are thought to have a major role in phytate-phosphorus cycling in soil and water. As their alternative name alkaline phytase suggests, BPPs work best in basic (or neutral) environment. Their pH optima is 6–9, which is unique among the phytases.











