Related Research Articles

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.
The peptidyl transferase is an aminoacyltransferase as well as the primary enzymatic function of the ribosome, which forms peptide bonds between adjacent amino acids using tRNAs during the translation process of protein biosynthesis. The substrates for the peptidyl transferase reaction are two tRNA molecules, one bearing the growing peptide chain and the other bearing the amino acid that will be added to the chain. The peptidyl chain and the amino acids are attached to their respective tRNAs via ester bonds to the O atom at the CCA-3' ends of these tRNAs. Peptidyl transferase is an enzyme that catalyzes the addition of an amino acid residue in order to grow the polypeptide chain in protein synthesis. It is located in the large ribosomal subunit, where it catalyzes the peptide bond formation. It is composed entirely of RNA. The alignment between the CCA ends of the ribosome-bound peptidyl tRNA and aminoacyl tRNA in the peptidyl transferase center contribute to its ability to catalyze these reactions. This reaction occurs via nucleophilic displacement. The amino group of the aminoacyl tRNA attacks the terminal carboxyl group of the peptidyl tRNA. Peptidyl transferase activity is carried out by the ribosome. Peptidyl transferase activity is not mediated by any ribosomal proteins but by ribosomal RNA (rRNA), a ribozyme. Ribozymes are the only enzymes which are not made up of proteins, but ribonucleotides. All other enzymes are made up of proteins. This RNA relic is the most significant piece of evidence supporting the RNA World hypothesis.
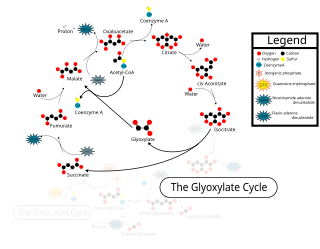
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
In enzymology, a peptidylglycine monooxygenase (EC 1.14.17.3) is an enzyme that catalyzes the chemical reaction
The enzyme diaminopropionate ammonia-lyase (EC 4.3.1.15) catalyzes the chemical reaction
The enzyme ureidoglycolate lyase (EC 4.3.2.3) catalyzes the chemical reaction
The enzyme L-erythro-3-hydroxyaspartate aldolase catalyzes the chemical reaction
The enzyme 4-hydroxy-2-oxoglutarate aldolase catalyzes the chemical reaction

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.
The enzyme malyl-CoA lyase catalyzes the chemical reaction

The enzyme methylisocitrate lyase catalyzes the chemical reaction
The enzyme oxalomalate lyase catalyzes the chemical reaction
The enzyme tartronate-semialdehyde synthase (EC 4.1.1.47) catalyzes the chemical reaction
In enzymology, a peptidyl-glutaminase (EC 3.5.1.43) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxyglutarate synthase (EC 2.3.3.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-ethylmalate synthase (EC 2.3.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-propylmalate synthase (EC 2.3.3.12) is an enzyme that catalyzes the chemical reaction

Peptidyl-glycine alpha-amidating monooxygenase is an enzyme that catalyzes the conversion of glycine amides to amides and glyoxylate.
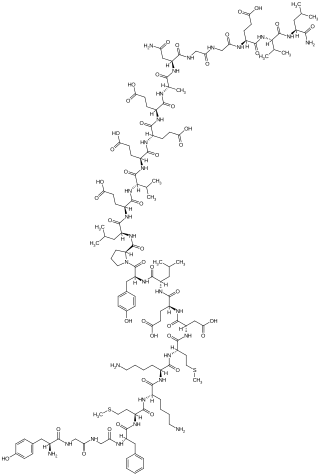
Amidorphin is an endogenous, C-terminally amidated, opioid peptide generated as a cleavage product of proenkephalin A in some mammalian species; in humans and most other species, the peptide is 1 residue longer and is not amidated. Amidorphin is widely distributed in the mammalian brain, with particularly high concentrations found in the striatum, and outside of the brain in adrenal medulla and posterior pituitary. The 26-residue peptide named amidorphin is found in several species including bovine, sheep, and pig. Humans and commonly studied lab animals produce a 27-residue peptide that does not have an amidated C-terminal residue; this is due to the absence of a Gly in the precursor sequence and replacement with Ala, which is not a substrate for the amidating enzyme. The properties of the 27-residue peptide are presumably similar to those of amidorphin, although this has not been adequately tested.
References
- Katopodis AG, Ping D, May SW (1990). "A novel enzyme from bovine neurointermediate pituitary catalyzes dealkylation of α-hydroxyglycine derivatives, thereby functioning sequentially with peptidylglycine α-amidating monooxygenase in peptide amidation". Biochemistry. 29 (26): 6115–20. doi:10.1021/bi00478a001. PMID 2207061.