Coniella fragariae is a plant pathogen. It is known to be pathogenic on eucalypts in a number of countries, including Brazil, India, China and Australia. In 2015, Coniella fragariae was reported as the causal agent for strawberry crown rot in Latvia. In 2018, the fungus was isolated from a goose dung collected in a strawberry field near the sea coast in North Germany. Inferred from the author, it should be a typical plant pathogenic fungus not coprophilous fungus. The plants, strawberry that had been eaten by geese are expected to be the true source of Coniella fragariae. Chemical constitution study showed azaphilone were the main secondary metabolites from this fungus.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 μg/mL, ranging from 3.5 (NEC) to 45 (A549) μg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 synchronized cells at 1-5 μg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.

Altechromone A is a chromone derivative. To date, it has been isolated from plant families such as Polygonaceae, Lamiaceae, Fabaceae, and Hypericaceae.
Aspergillus sydowii is a pathogenic fungus that causes several diseases in humans. It has been implicated in the death of sea fan corals in the Caribbean Sea.

Secalonic acids are a group of xanthone derivatives closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses. From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified. Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.

Phomopsis longicolla is a species of ascomycete fungus in the family Diaporthaceae. It is a plant pathogen and mainly responsible for a soybean disease called Phomopsis seed decay (PSD). In other plant species, P. longicolla can also live as an endophyte, such as in the mangrove plant Sonneratia caseolaris. P. longicolla has been found to produce a number of cytotoxic and antimicrobial secondary metabolites, especially members of the class of phomoxanthones. P. longicolla was first described in 1985 by Thomas W. Hobbs et al. at the Department of Plant Pathology at Ohio State University.

Stephacidin A and B are antitumor alkaloids isolated from the fungus Aspergillus ochraceus that belong to a class of naturally occurring 2,5-diketopiperazines. This unusual family of fungal metabolites are complex bridged 2,5-diketopiperazine alkaloids that possess a unique bicyclo[2.2.2]diazaoctane core ring system and are constituted mainly from tryptophan, proline, and substituted proline derivatives where the olefinic unit of the isoprene moiety has been formally oxidatively cyclized across the α-carbon atoms of a 2,5-diketopiperazine ring. The molecular architecture of stephacidin B, formally a dimer of avrainvillamide, reveals a complex dimeric prenylated N-hydroxyindole alkaloid that contains 15 rings and 9 stereogenic centers and is one of the most complex indole alkaloids isolated from fungi. Stephacidin B rapidly converts into the electrophilic monomer avrainvillamide in cell culture, and there is evidence that the monomer avrainvillamide interacts with intracellular thiol-containing proteins, most likely by covalent modification.

14-Norpseurotin A is an alkaloid and a bio-active metabolite of Aspergillus, featuring an oxa-spiro-lactam core.
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Penicillium brocae is a fungal species of the genus Penicillium, which was isolated in Chiapas in Mexico. It is a symbiont of the mangrove tree Avicennia marina.
Penicillium paneum is a species of fungus in the genus Penicillium which can spoil cereal grains. Penicillium paneum produces 1-Octen-3-ol and penipanoid A, penipanoid B, penipanoid C, patulin and roquefortine C
Penicillium vinaceum is an anamorph species of fungus in the genus Penicillium which produces penicillivinacine, vinaxanthone and citromycetin.

Anicequol is a naturally occurring ergostane steroid produced by Acremonium sp. TF-0356 which has nerve growth factor-like neurotrophic activity. It was under investigation by Taisho Pharmaceutical in Japan for the treatment of cognitive disorders in the 1990s, but development was discontinued and the drug was never marketed.

The mycotoxin phomoxanthone A, or PXA for short, is a toxic natural product that affects the mitochondria. It is the most toxic and the best studied of the naturally occurring phomoxanthones. PXA has recently been shown to induce rapid, non-canonical mitochondrial fission by causing the mitochondrial matrix to fragment while the outer mitochondrial membrane can remain intact. This process was shown to be independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
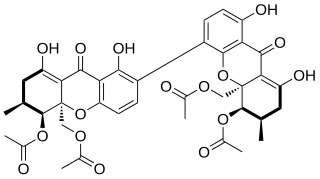
The mycotoxin phomoxanthone B, or PXB for short, is a toxic natural product. It is a less toxic isomer of phomoxanthone A and one of the two founding members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. PXB itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between PXB and its isomers PXA and dicerandrol C: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.
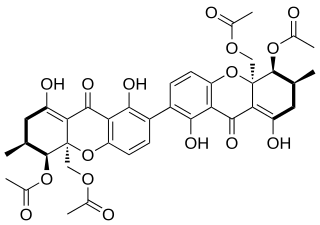
Dicerandrol C is a natural product. It is a less toxic isomer of phomoxanthone A (PXA) and phomoxanthone B (PXB), all three of which are members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. Dicerandrol C itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between dicerandrol C and its isomers PXA and PXB: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.

Tetrahydroxanthones are natural products formally derived by partial reduction of xanthone. They are produced by various fungi, bacteria, and plants. Some are precursors to larger xanthone natural products. One example is neosartorin, composed of 5-acetylblennolide A and blennolide C, exhibits antibacterial activity against Gram-positive bacteria, notably including Staphylococcus aureus.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.
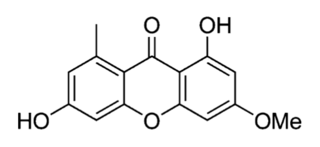
Griseoxanthone C is an organic compound in the structural class of chemicals known as xanthones. Its chemical formula is 1,6-dihydroxy-3-methoxy-8-methylxanthen-9-one, and its molecular formula is C15H12O5. It is found in a plant and some fungi, including a lichen.
















