In chemistry, a zwitterion, also called an inner salt, is a molecule that contains an equal number of positively- and negatively-charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.
Cubane (C8H8) is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, researchers believed that cubic carbon-based molecules would be too unstable to exist. The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.

Catechin is a flavan-3-ol, a type of natural phenol and antioxidant. It is a plant secondary metabolite. It belongs to the group of flavan-3-ols, part of the chemical family of flavonoids.
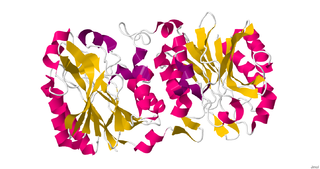
Nitrilase enzymes catalyse the hydrolysis of nitriles to carboxylic acids and ammonia, without the formation of "free" amide intermediates. Nitrilases are involved in natural product biosynthesis and post translational modifications in plants, animals, fungi and certain prokaryotes. Nitrilases can also be used as catalysts in preparative organic chemistry. Among others, nitrilases have been used for the resolution of racemic mixtures. Nitrilase should not be confused with nitrile hydratase which hydrolyses nitriles to amides. Nitrile hydratases are almost invariably co-expressed with an amidase, which converts the amide to the carboxylic acid. Consequently, it can sometimes be difficult to distinguish nitrilase activity from nitrile hydratase plus amidase activity.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
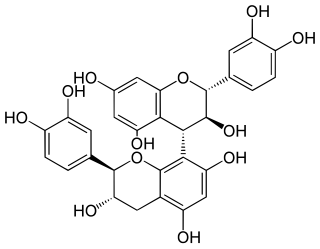
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 µg/mL, ranging from 3.5 (NEC) to 45 (A549) µg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 cynchronized cells at 1-5 µg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures and wine. It can also be found in Rheum maximowiczii.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Secalonic acids are a group of chiral dimeric tetrahydroxanthones closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses. From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified. Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.

Phomopsis longicolla is a species of ascomycete fungus in the family Diaporthaceae. It is a plant pathogen and mainly responsible for a soybean disease called Phomopsis seed decay (PSD). In other plant species, P. longicolla can also live as an endophyte, such as in the mangrove plant Sonneratia caseolaris. P. longicolla has also been found to produce a number of cytotoxic and antimicrobial secondary metabolites, especially members of the class of phomoxanthones. P. longicolla was first described in 1985 by Thomas W. Hobbs et al. at the Department of Plant Pathology at Ohio State University.
Penicillium herquei is an anamorph, filamentous species of the genus of Penicillium which produces citreorosein, emodin, hualyzin, herquline B, janthinone, citrinin and duclauxin,.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.

Brevianamide F , also known as cyclo-(L-Trp-L-Pro), belongs to a class of naturally occurring 2,5-diketopiperazines. It is the simplest member and the biosynthetic precursor of a large family of biologically active prenylated tryptophan-proline 2,5-diketopiperazines that are produced by the fungi A. fumigates and Aspergillus sp. It has been isolated from the bacterium Streptomyces sp. strain TN58 and shown to possess activity against the Gram-positive bacteria S. aureus and Micrococcus luteus. It has also been isolated from Bacillus cereus associated with the entomopathogenic nematode Rhabditis (Oscheius) sp. and shown to have antifungal activity against T. rubrum, C. neoformans, and C. albicans, better than amphotericin B. Although the proline 2,5-diketopiperazines are the most abundant and structurally diverse 2,5-diketopiperazines found in food, cyclo(L-Trp-L-Pro) has only been found as a minor 2,5-diketopiperazine (8.2 ppm) in autolyzed yeast extract. Initially, cyclo(L-Trp-L-Pro) and its DL, LD, and DD isomers showed potential for use in the treatment of cardiovascular dysfunction, but they were later shown to be hepatotoxic.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.

The mycotoxin phomoxanthone A, or PXA for short, is a toxic natural product that affects the mitochondria. It is the most toxic and the best studied of the naturally occurring phomoxanthones. PXA has recently been shown to induce rapid, non-canonical mitochondrial fission by causing the mitochondrial matrix to fragment while the outer mitochondrial membrane can remain intact. This process was shown to be independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
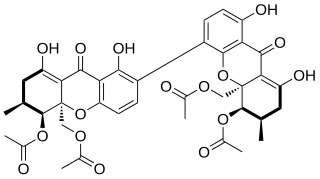
The mycotoxin phomoxanthone B, or PXB for short, is a toxic natural product. It is a less toxic isomer of phomoxanthone A and one of the two founding members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. PXB itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between PXB and its isomers PXA and dicerandrol C: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.
The molecular formula C38H38O16 (molar mass: 750.70 g/mol, exact mass: 750.2160 u) may refer to:














