
Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in organic chemistry. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing sides. Cis-trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis-trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. In general, stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

Trinitrotoluene more commonly known as TNT, or more specifically 2,4,6-trinitrotoluene, is a chemical compound with the formula C6H2(NO2)3CH3. This yellow solid is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.
XScale is a microarchitecture for central processing units initially designed by Intel implementing the ARM architecture instruction set. XScale comprises several distinct families: IXP, IXC, IOP, PXA and CE, with some later models designed as SoCs. Intel sold the PXA family to Marvell Technology Group in June 2006. Marvell then extended the brand to include processors with other microarchitectures, like ARM's Cortex.
Chlordane, or chlordan, is an organochlorine compound used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.

An excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons. In this case, formation of molecules is possible only if such atom is in an electronic excited state. Heteronuclear molecules and molecules that have more than two species are also called exciplex molecules. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is very short, on the order of nanoseconds. Binding of a larger number of excited atoms forms Rydberg matter clusters, the lifetime of which can exceed many seconds.
Cubane (C8H8) is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, researchers believed that cubic carbon-based molecules would be too unstable to exist. The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
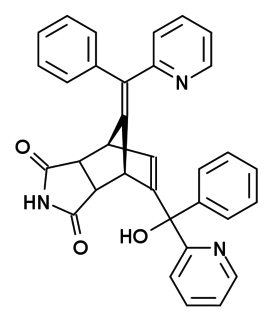
Norbormide is a toxic compound used as a rodenticide. It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker, but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 µg/mL, ranging from 3.5 (NEC) to 45 (A549) µg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 cynchronized cells at 1-5 µg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.

Secalonic acids are a group of chiral dimeric tetrahydroxanthones closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses. From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified. Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.

Phomopsis longicolla is a species of ascomycete fungus in the family Diaporthaceae. It is a plant pathogen and mainly responsible for a soybean disease called Phomopsis seed decay (PSD). In other plant species, P. longicolla can also live as an endophyte, such as in the mangrove plant Sonneratia caseolaris. P. longicolla has also been found to produce a number of cytotoxic and antimicrobial secondary metabolites, especially members of the class of phomoxanthones. P. longicolla was first described in 1985 by Thomas W. Hobbs et al. at the Department of Plant Pathology at Ohio State University.

Methylthioirontricarbonyl dimer, also known as methanethiolatoirontricarbonyl dimer, is an organometallic compound with the formula Fe2(SCH3)2(CO)6. It is a red volatile solid that is classified as a transition metal thiolate complex. It exists as air-stable red crystals with two isomers, where the methyl groups are either anti (isomer A) or syn (isomer B) with respect to each other.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.

The mycotoxin phomoxanthone A, or PXA for short, is a toxic natural product that affects the mitochondria. It is the most toxic and the best studied of the naturally occurring phomoxanthones. PXA has recently been shown to induce rapid, non-canonical mitochondrial fission by causing the mitochondrial matrix to fragment while the outer mitochondrial membrane can remain intact. This process was shown to be independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
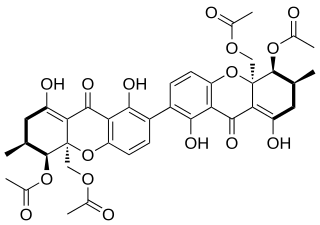
Dicerandrol C is a natural product. It is a less toxic isomer of phomoxanthone A (PXA) and phomoxanthone B (PXB), all three of which are members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. Dicerandrol C itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between dicerandrol C and its isomers PXA and PXB: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.

Tetrahydroxanthones are natural products formally derived by partial reduction of xanthone. They are produced by various fungi, bacteria, and plants. Some are precursors to larger xanthone natural products. One example is neosartorin, composed of 5-acetylblennolide A and blennolide C, exhibits antibacterial activity against Gram-positive bacteria, notably including Staphylococcus aureus.















