Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades.
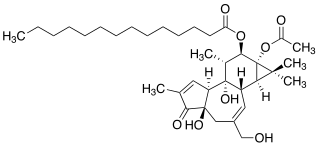
12-O-Tetradecanoylphorbol-13-acetate (TPA), also commonly known as tetradecanoylphorbol acetate, tetradecanoyl phorbol acetate, and phorbol 12-myristate 13-acetate (PMA), is a diester of phorbol and a potent tumor promoter often employed in biomedical research to activate the signal transduction enzyme protein kinase C (PKC). The effects of TPA on PKC result from its similarity to one of the natural activators of classic PKC isoforms, diacylglycerol. TPA is a small molecule drug.

Croton is an extensive flowering plant genus in the spurge family, Euphorbiaceae. The plants of this genus were described and introduced to Europeans by Georg Eberhard Rumphius. The common names for this genus are rushfoil and croton, but the latter also refers to Codiaeum variegatum. The generic name comes from the Greek κρότος, which means "tick" and refers to the shape of the seeds of certain species.
Croton oil is an oil prepared from the seeds of Croton tiglium, a tree belonging to the order Euphorbiales and family Euphorbiaceae, and native or cultivated in India and the Malay Archipelago. Small doses taken internally cause diarrhea. Externally, the oil can cause irritation and swelling. Croton oil is used in Phenol-croton oil chemical peels for its caustic exfoliating effects it has on the skin. Used in conjunction with phenol solutions, it results in an intense reaction which leads to initial skin sloughing. Since croton oil is very irritating and painful, it is used in laboratory animals to study how pain works, pain-relieving and anti-inflammatory drugs, and immunology.

Phorbol is a natural, plant-derived organic compound. It is a member of the tigliane family of diterpenes. Phorbol was first isolated in 1934 as the hydrolysis product of croton oil, which is derived from the seeds of the purging croton, Croton tiglium. The structure of phorbol was determined in 1967. Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C. They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with fatty acids to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis.

Phorbol esters are a class of chemical compounds found in a variety of plants, particularly in the families Euphorbiaceae and Thymelaeaceae. Chemically, they are ester derivatives of the tetracyclic diterpenoid phorbol.
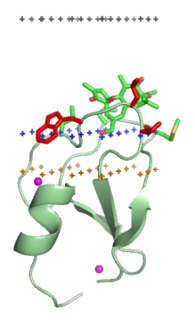
C1 domain binds an important secondary messenger diacylglycerol (DAG), as well as the analogous phorbol esters. Phorbol esters can directly stimulate protein kinase C, PKC. The N-terminal region of PKC, known as C1, has been shown

Protein kinase C delta type is an enzyme that in humans is encoded by the PRKCD gene.

Protein kinase C beta type is an enzyme that in humans is encoded by the PRKCB gene.
Protein kinase C gamma type is an enzyme that in humans is encoded by the PRKCG gene.
In enzymology, a phorbol-diester hydrolase (EC 3.1.1.51) is an enzyme that catalyzes the chemical reaction

Aplysiatoxin is a cyanotoxin produced by certain cyanobacteria species. It is used as a defensive secretion to protect these cyanobacteria from predation by fish, being a potent irritant and carcinogen, by acting as a powerful activator of protein kinase C. While this action has a tumour-promoting effect, protein kinase C activation can be medically beneficial for some other applications, and synthetic analogues of aplysiatoxin have been researched for anti-cancer effects.

Protein kinase C eta type is an enzyme that in humans is encoded by the PRKCH gene.

Beta-chimaerin is a protein that in humans is encoded by the CHN2 gene.
The molecular formula C28H40O8 (molar mass: 504.61 g/mol) may refer to:
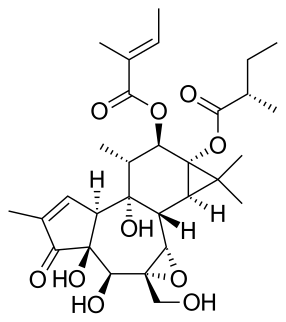
Tigilanol tiglate, sold under the brand name Stelfonta is a medication used to treat dogs with non-metastatic, skin-based (cutaneous) mast cell tumors (MCTs). The FDA is also approving Stelfonta to treat non-metastatic MCTs located under the dog’s skin (subcutaneous), in particular areas of a dog’s leg. Stelfonta is injected directly into the MCT. Stelfonta works by activating a protein that spreads throughout the treated tumor, which disintegrates tumor cells.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.

Mezerein is a toxic diterpene ester found in the sap of Daphne mezereum and related plants. Plants of the genera Euphorbiaceae and Thymelaeaceae possess a wide variety of different phorbol esters, which share the capacity of mimicking diacylglycerol (DAG) and thus activating different isoforms of protein kinase C. Mezerein was first isolated in 1975. It has antileukemic properties in mice, but it is also defined as a weak promoter of skin cancers in the same species. All parts of the plants contain an acrid and irritant sap that contains mezerein, thought to be the principal poison. The sap is especially prevalent in the bark and berries.
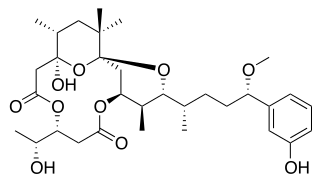
Debromoaplysiatoxin is a toxic agent produced by the blue-green alga Lyngbya majuscula. This alga lives in marine waters and causes seaweed dermatitis. Furthermore, it is a tumor promoter which has an anti-proliferative activity against various cancer cell lines in mice.
Karen L. Leach is an American biochemist with extensive drug discovery experience in large pharmaceutical research laboratories. Her expertise in molecular pharmacology, signal transduction and protein kinases, has been used to establish mechanisms of toxicity for therapeutics such as the novel antibiotic linezolid (Zyvox).














