Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant [1] and in the plasma membrane where it directly activates lipid-gated ion channels.
Contents
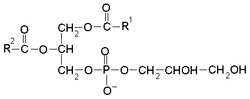
The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. It is the precursor of surfactant and its presence (>0.3) in the amniotic fluid of the newborn indicates fetal lung maturity.
Approximately 98% of alveolar wall surface area is due to the presence of type I cells, with type II cells producing pulmonary surfactant covering around 2% of the alveolar walls. Once surfactant is secreted by the type II cells, it must be spread over the remaining type I cellular surface area. Phosphatidylglycerol is thought to be important in spreading of surfactant over the Type I cellular surface area. The major surfactant deficiency in premature infants relates to the lack of phosphatidylglycerol, even though it comprises less than 5% of pulmonary surfactant phospholipids. It is synthesized by head group exchange of a phosphatidylcholine enriched phospholipid using the enzyme phospholipase D.
