
The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.

Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.

Phosphatidylinositol or inositol phospholipid is a biomolecule. It was initially called "inosite" when it was discovered by Léon Maquenne and Johann Joseph von Scherer in the late 19th century. It was discovered in bacteria but later also found in eukaryotes, and was found to be a signaling molecule.
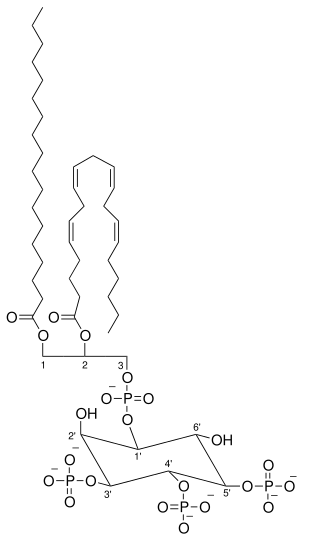
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.
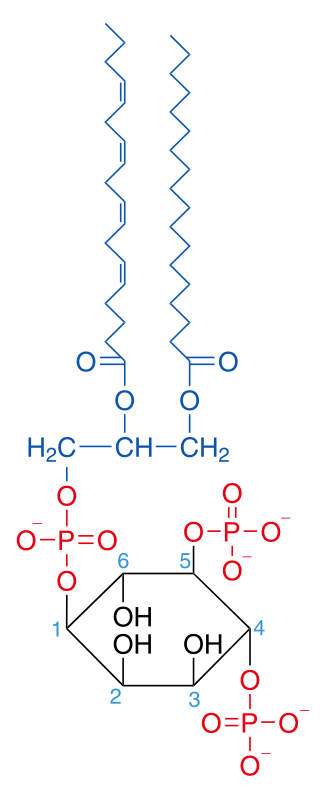
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Phosphatidylinositol 3-phosphate (PI3P) is a phospholipid found in cell membranes that helps to recruit a range of proteins, many of which are involved in protein trafficking, to the membranes. It is the product of both the class II and III phosphoinositide 3-kinases activity on phosphatidylinositol.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

Pleckstrin homology domain or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton.

Phosphatidylinositol 3,5-bisphosphate is one of the seven phosphoinositides found in eukaryotic cell membranes. In quiescent cells, the PtdIns(3,5)P2 levels, typically quantified by HPLC, are the lowest amongst the constitutively present phosphoinositides. They are approximately 3 to 5-fold lower as compared to PtdIns3P and PtdIns5P levels, and more than 100-fold lower than the abundant PtdIns4P and PtdIns(4,5)P2. PtdIns(3,5)P2 was first reported to occur in mouse fibroblasts and budding yeast S. cerevisiae in 1997. In S. cerevisiae PtdIns(3,5)P2 levels increase dramatically during hyperosmotic shock. The response to hyperosmotic challenge is not conserved in most tested mammalian cells except for differentiated 3T3L1 adipocytes.
Phospholipase D (EC 3.1.4.4, lipophosphodiesterase II, lecithinase D, choline phosphatase, PLD; systematic name phosphatidylcholine phosphatidohydrolase) is an anesthetic sensitive and mechanosensitive enzyme of the phospholipase superfamily that catalyses the following reaction

Phosphatidylinositol 4-kinase beta is an enzyme that in humans is encoded by the PI4KB gene.

PIKfyve, a FYVE finger-containing phosphoinositide kinase, is an enzyme that in humans is encoded by the PIKFYVE gene.

Collagen type IV alpha-3-binding protein, also known as ceramide transfer protein (CERT) or StAR-related lipid transfer protein 11 (STARD11) is a protein that in humans is encoded by the COL4A3BP gene. The protein contains a pleckstrin homology domain at its amino terminus and a START domain towards the end of the molecule. It is a member of the StarD2 subfamily of START domain proteins.

Oxysterol-binding protein 1 is a protein that in humans is encoded by the OSBP gene.

Phosphatidylinositol 4-kinase 2-alpha is an enzyme that in humans is encoded by the PI4K2A gene.

Zinc finger FYVE domain-containing protein 1 is a protein that in humans is encoded by the ZFYVE1 gene.
Phosphatidylinositol 5-phosphate (PtdIns5P) is a phosphoinositide, one of the phosphorylated derivatives of phosphatidylinositol (PtdIns), that are well-established membrane-anchored regulatory molecules. Phosphoinositides participate in signaling events that control cytoskeletal dynamics, intracellular membrane trafficking, cell proliferation and many other cellular functions. Generally, phosphoinositides transduce signals by recruiting specific phosphoinositide-binding proteins to intracellular membranes.

Sec14 is a cytosolic protein found in yeast which plays a role in the regulation of several cellular functions, specifically those related to intracellular transport. Encoded by the Sec14 gene, Sec14p may transport phosphatidylinositol and phosphatidylcholine produced in the endoplasmic reticulum and the Golgi body to other cellular membranes. Additionally, Sec14p potentially plays a role in the localization of lipid raft proteins. Sec14p is an essential gene in yeast, and is homologous in function to phosphatidylinositol transfer protein in mammals. A conditional mutant with non-functional Sec14p presents with Berkeley bodies and deficiencies in protein secretion.

The omegasome is a cell organelle consisting of lipid bilayer membranes enriched for phosphatidylinositol 3-phosphate, and related to a process of autophagy. It is a subdomain of the endoplasmic reticulum (ER), and has a morphology resembling the Greek capital letter Omega (Ω). Omegasomes are the sites from which phagophores form, which are sack-like structures that mature into autophagosomes, and fuse with lysosomes in order to degrade the contents of the autophagosomes. The formation of omegasomes depends on various factors, however in general, formation of omegasomes is increased as a response to starvation, and in some biochemical situations the presence of PI(3)P leads to the formation of omegasomes.


















