Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.

Phosphatidylinositol or inositol phospholipid is a biomolecule. It was initially called "inosite" when it was discovered by Léon Maquenne and Johann Joseph von Scherer in the late 19th century. It was discovered in bacteria but later also found in eukaryotes, and was found to be a signaling molecule.

Phosphoinositide phospholipase C is a family of eukaryotic intracellular enzymes that play an important role in signal transduction processes. These enzymes belong to a larger superfamily of Phospholipase C. Other families of phospholipase C enzymes have been identified in bacteria and trypanosomes. Phospholipases C are phosphodiesterases.
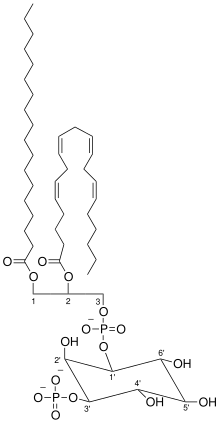
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.
Class III PI 3-kinase is a subgroup of the enzyme family, phosphoinositide 3-kinase that share a common protein domain structure, substrate specificity and method of activation.
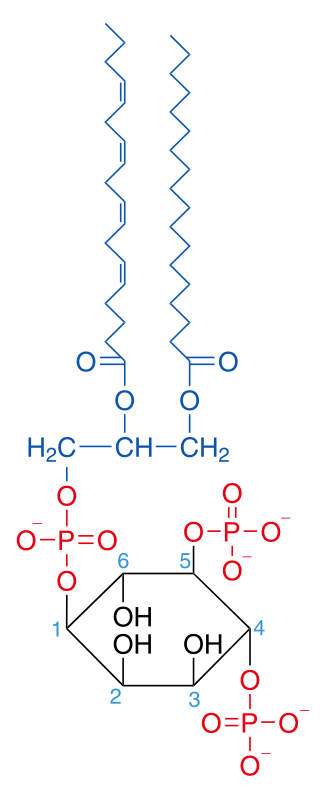
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

Pleckstrin homology domain or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton.

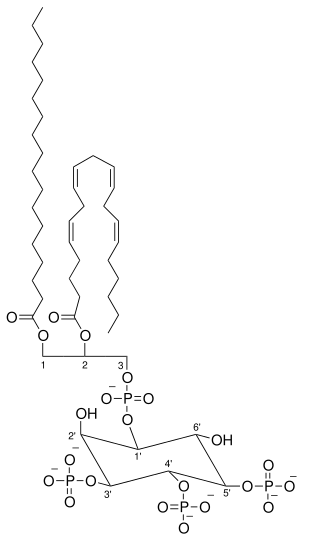
Phosphatidylinositol 3,5-bisphosphate is one of the seven phosphoinositides found in eukaryotic cell membranes. In quiescent cells, the PtdIns(3,5)P2 levels, typically quantified by HPLC, are the lowest amongst the constitutively present phosphoinositides. They are approximately 3 to 5-fold lower as compared to PtdIns3P and PtdIns5P levels, and more than 100-fold lower than the abundant PtdIns4P and PtdIns(4,5)P2. PtdIns(3,5)P2 was first reported to occur in mouse fibroblasts and budding yeast S. cerevisiae in 1997. In S. cerevisiae PtdIns(3,5)P2 levels increase dramatically during hyperosmotic shock. The response to hyperosmotic challenge is not conserved in most tested mammalian cells except for differentiated 3T3L1 adipocytes.
In molecular biology the FYVE zinc finger domain is named after the four cysteine-rich proteins: Fab 1, YOTB, Vac 1, and EEA1, in which it has been found. FYVE domains bind phosphatidylinositol 3-phosphate, in a way dependent on its metal ion coordination and basic amino acids. The FYVE domain inserts into cell membranes in a pH-dependent manner. The FYVE domain has been connected to vacuolar protein sorting and endosome function.
The PX domain is a phosphoinositide-binding structural domain involved in targeting of proteins to cell membranes.
Phosphatidylinositol-4-phosphate is a precursor of phosphatidylinositol (4,5)-bisphosphate. PtdIns4P is prevalent in the membrane of the Golgi apparatus.

Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing alpha polypeptide is an enzyme that in humans is encoded by the PIK3C2A gene.

Phosphatidylinositol 4-kinase beta is an enzyme that in humans is encoded by the PI4KB gene.

PIKfyve, a FYVE finger-containing phosphoinositide kinase, is an enzyme that in humans is encoded by the PIKFYVE gene.

Phosphatidylinositol 4-kinase 2-alpha is an enzyme that in humans is encoded by the PI4K2A gene.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.
Phosphatidylinositol 5-phosphate (PtdIns5P) is a phosphoinositide, one of the phosphorylated derivatives of phosphatidylinositol (PtdIns), that are well-established membrane-anchored regulatory molecules. Phosphoinositides participate in signaling events that control cytoskeletal dynamics, intracellular membrane trafficking, cell proliferation and many other cellular functions. Generally, phosphoinositides transduce signals by recruiting specific phosphoinositide-binding proteins to intracellular membranes.

Protein VAC14 homolog, also known as ArPIKfyve, is a protein that in humans is encoded by the VAC14 gene.