
Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses - specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. Non-animals like plants and fungi do not have nerve cells.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

Guanylate cyclase is a lyase enzyme that converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate:
Behavioral neuroscience, also known as biological psychology, biopsychology, or psychobiology, is the application of the principles of biology to the study of physiological, genetic, and developmental mechanisms of behavior in humans and other animals.
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes. Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions have been cloned from other species of algae and protists.
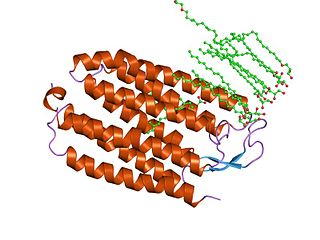
Halorhodopsin is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is a chloride-specific light-gated ion pump found in archaea known as halobacteria. It is activated by green light wavelengths of approximately 578nm. Halorhodopsin also shares sequence similarity to channelrhodopsin, another light-driven ion channel.
Retinylidene proteins, or rhodopsins in a broad sense, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.
Light-gated ion channels are a family of ion channels regulated by electromagnetic radiation. Other gating mechanisms for ion channels include voltage-gated ion channels, ligand-gated ion channels, mechanosensitive ion channels, and temperature-gated ion channels. Most light-gated ion channels have been synthesized in the laboratory for study, although two naturally occurring examples, channelrhodopsin and anion-conducting channelrhodopsin, are currently known. Photoreceptor proteins, which act in a similar manner to light-gated ion channels, are generally classified instead as G protein-coupled receptors.

Gero Andreas Miesenböck is an Austrian scientist. He is currently Waynflete Professor of Physiology and Director of the Centre for Neural Circuits and Behaviour (CNCB) at the University of Oxford and a fellow of Magdalen College, Oxford.

Opsin-3 also known as encephalopsin or panopsin is a protein that, in humans, is encoded by the OPN3 gene. Alternative splicing of this gene results in multiple transcript variants encoding different protein isoforms.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient.

Karl Alexander Deisseroth is an American scientist. He is the D.H. Chen Professor of Bioengineering and of psychiatry and behavioral sciences at Stanford University.

Photoactivatable probes, or caged probes, are cellular players that can be triggered by a flash of light. They are used in biological research to study processes in cells. The basic principle is to bring a photoactivatable agent to cells, tissues or even living animals and specifically control its activity by illumination.
Gene therapy is being studied for some forms of epilepsy. It relies on viral or non-viral vectors to deliver DNA or RNA to target brain areas where seizures arise, in order to prevent the development of epilepsy or to reduce the frequency and/or severity of seizures. Gene therapy has delivered promising results in early stage clinical trials for other neurological disorders such as Parkinson's disease, raising the hope that it will become a treatment for intractable epilepsy.
Chemogenetics is the process by which macromolecules can be engineered to interact with previously unrecognized small molecules. Chemogenetics as a term was originally coined to describe the observed effects of mutations on chalcone isomerase activity on substrate specificities in the flowers of Dianthus caryophyllus. This method is very similar to optogenetics; however, it uses chemically engineered molecules and ligands instead of light and light-sensitive channels known as opsins.

Peter Hegemann is a Hertie Senior Research Chair for Neurosciences and a Professor of Experimental Biophysics at the Department of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany. He is known for his discovery of channelrhodopsin, a type of ion channels regulated by light, thereby serving as a light sensor. This created the field of optogenetics, a technique that controls the activities of specific neurons by applying light. He has received numerous accolades, including the Rumford Prize, the Shaw Prize in Life Science and Medicine, and the Albert Lasker Award for Basic Medical Research.
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point for retinal.

Anion-conducting channelrhodopsins are light-gated ion channels that open in response to light and let negatively charged ions enter a cell. All channelrhodopsins use retinal as light-sensitive pigment, but they differ in their ion selectivity. Anion-conducting channelrhodopsins are used as tools to manipulate brain activity in mice, fruit flies and other model organisms (Optogenetics). Neurons expressing anion-conducting channelrhodopsins are silenced when illuminated with light, an effect that has been used to investigate information processing in the brain. For example, suppressing dendritic calcium spikes in specific neurons with light reduced the ability of mice to perceive a light touch to a whisker. Studying how the behavior of an animal changes when specific neurons are silenced allows scientists to determine the role of these neurons in the complex circuits controlling behavior.

Georg Nagel is a biophysicist and professor at the Department for Neurophysiology at the University of Würzburg in Germany. His research is focused on microbial photoreceptors and the development of optogenetic tools.
Lisa Gunaydin is an American neuroscientist and assistant professor at the Weill Institute for Neurosciences at the University of California San Francisco. Gunaydin helped discover optogenetics in the lab of Karl Deisseroth and now uses this technique in combination with neural and behavioral recordings to probe the neural circuits underlying emotional behaviors.













