
Bacteriorhodopsin (Bop) is a protein used by Archaea, most notably by haloarchaea, a class of the Euryarchaeota. It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy.

Behavioral neuroscience, also known as biological psychology, biopsychology, or psychobiology, is the application of the principles of biology to the study of physiological, genetic, and developmental mechanisms of behavior in humans and other animals.

Proteorhodopsin is a family of transmembrane proteins that use retinal as a chromophore for light-mediated functionality, in this case, a proton pump. pRhodopsin is found in marine planktonic bacteria, archaea and eukaryotes (protae), but was first discovered in bacteria.
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes. Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions have been cloned from other species of algae and protists.

Photostimulation is the use of light to artificially activate biological compounds, cells, tissues, or even whole organisms. Photostimulation can be used to noninvasively probe various relationships between different biological processes, using only light. In the long run, photostimulation has the potential for use in different types of therapy, such as migraine headache. Additionally, photostimulation may be used for the mapping of neuronal connections between different areas of the brain by “uncaging” signaling biomolecules with light. Therapy with photostimulation has been called light therapy, phototherapy, or photobiomodulation.
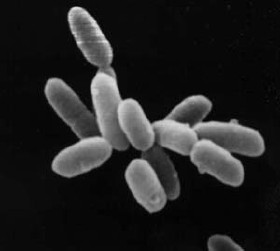
Halobacterium is a genus in the family Halobacteriaceae.

The Kir2.1 inward-rectifier potassium channel is a lipid-gated ion channel encoded by the KCNJ2 gene.
Retinylidene proteins, or rhodopsins in a broad sense, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.
Light-gated ion channels are a family of ion channels regulated by electromagnetic radiation. Other gating mechanisms for ion channels include voltage-gated ion channels, ligand-gated ion channels, mechanosensitive ion channels, and temperature-gated ion channels. Most light-gated ion channels have been synthesized in the laboratory for study, although two naturally occurring examples, channelrhodopsin and anion-conducting channelrhodopsin, are currently known. Photoreceptor proteins, which act in a similar manner to light-gated ion channels, are generally classified instead as G protein-coupled receptors.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient.

Edward S. Boyden is an American neuroscientist at MIT. He is the Y. Eva Tan Professor in Neurotechnology, a faculty member in the MIT Media Lab and an associate member of the McGovern Institute for Brain Research. In 2018 he was named a Howard Hughes Medical Institute Investigator. He is recognized for his work on optogenetics. In this technology, a light-sensitive ion channel such as channelrhodopsin-2 is genetically expressed in neurons, allowing neuronal activity to be controlled by light. There were early efforts to achieve targeted optical control dating back to 2002 that did not involve a directly light-activated ion channel, but it was the method based on directly light-activated channels from microbes, such as channelrhodopsin, emerging in 2005 that turned out to be broadly useful. Optogenetics in this way has been widely adopted by neuroscientists as a research tool, and it is also thought to have potential therapeutic applications. Boyden joined the MIT faculty in 2007, and continues to develop new optogenetic tools as well as other technologies for the manipulation of brain activity. Previously, Boyden received degrees in electrical engineering, computer science, and physics from MIT. During high school, Boyden attended the Texas Academy of Mathematics and Science.

Karl Alexander Deisseroth is an American scientist. He is the D.H. Chen Professor of Bioengineering and of psychiatry and behavioral sciences at Stanford University.

Peter Hegemann is a Hertie Senior Research Chair for Neurosciences and a Professor of Experimental Biophysics at the Department of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany. He is known for his discovery of channelrhodopsin, a type of ion channels regulated by light, thereby serving as a light sensor. This created the field of optogenetics, a technique that controls the activities of specific neurons by applying light. He has received numerous accolades, including the Rumford Prize, the Shaw Prize in Life Science and Medicine, and the Albert Lasker Award for Basic Medical Research.
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point for retinal.

Anion-conducting channelrhodopsins are light-gated ion channels that open in response to light and let negatively charged ions enter a cell. All channelrhodopsins use retinal as light-sensitive pigment, but they differ in their ion selectivity. Anion-conducting channelrhodopsins are used as tools to manipulate brain activity in mice, fruit flies and other model organisms (Optogenetics). Neurons expressing anion-conducting channelrhodopsins are silenced when illuminated with light, an effect that has been used to investigate information processing in the brain. For example, suppressing dendritic calcium spikes in specific neurons with light reduced the ability of mice to perceive a light touch to a whisker. Studying how the behavior of an animal changes when specific neurons are silenced allows scientists to determine the role of these neurons in the complex circuits controlling behavior.

Herwig Baier is a German neurobiologist with dual German and US-American citizenship. He is Director at the Max Planck Institute for Biological Intelligence and head of the department Genes – Circuits – Behavior. Herwig Baier's research aims to understand how animal brains convert sensory inputs into behavioral responses.

Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian GPCR protein rhodopsin, but are not true homologs.

Georg Nagel is a biophysicist and professor at the Department for Neurophysiology at the University of Würzburg in Germany. His research is focused on microbial photoreceptors and the development of optogenetic tools.
Lisa Gunaydin is an American neuroscientist and assistant professor at the Weill Institute for Neurosciences at the University of California San Francisco. Gunaydin helped discover optogenetics in the lab of Karl Deisseroth and now uses this technique in combination with neural and behavioral recordings to probe the neural circuits underlying emotional behaviors.
Dieter Oesterhelt was a German biochemist. From 1980 until 2008, he was director of the Max Planck Institute for Biochemistry, Martinsried.














