
Tin is a chemical element; it has symbol Sn and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of twinning in tin crystals.

A pigment is a powder used to add color or change visual appearance. Pigments are completely or nearly insoluble and chemically unreactive in water or another medium; in contrast, dyes are colored substances which are soluble or go into solution at some stage in their use. Dyes are often organic compounds whereas pigments are often inorganic. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4", as a phonetic representation of the symbols of its molecular formula.

Azo compounds are organic compounds bearing the functional group diazenyl.
Chrome yellow is a bright, warm yellow pigment that has been used in art, fashion, and industry. It is the premier orange pigment for many applications.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
Arylide yellow, also known as Hansa yellow and monoazo yellow, is a family of organic compounds used as pigments. They are primarily used as industrial colorants including plastics, building paints and inks. They are also used in artistic oil paints, acrylics and watercolors. These pigments are usually semi-transparent and range from orange-yellow to yellow-greens. Related organic pigments are the diarylide pigments. Overall, these pigments have partially displaced the toxic cadmium yellow in the marketplace. Painters such as Alexander Calder and Jackson Pollock are known to have employed arylide yellow in their artworks.

In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally. Isoindoles units occur in phthalocyanines, an important family of dyes. Some alkaloids containing isoindole have been isolated and characterized.

3,3'-Dichlorobenzidine is an organic compound with the formula (C6H3Cl(NH2))2. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of diarylide yellow pigments used in the production of printing inks. Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity.

Phthalonitrile is an organic compound with the formula C6H4(CN)2, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers.
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. Being pigments, these compounds exist as (yellow) powders of low solubility in water. They are similar to the simpler monoazo pigments called arylide yellows.

Pigment Yellow 16 is an organic compound that is classified as a diarylide pigment.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74.
Pigment Yellow 83 is an organic compound that is classified as a diarylide pigment. It is used as a yellow colorant.
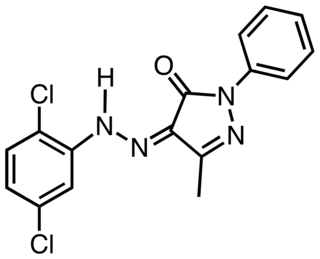
Pigment Yellow 10 is an organic compound that is classified as a monoazopyrazolone pigment. It is used as a yellow colorant, notably as yellow road marking on highways in the US.

Pigment yellow 139 is an organic compound that is used as a yellow-orange pigment. It is classified as a derivative of isoindoline. This yellow-orange solid is virtually insoluble in most solvents.

Pigment yellow 185 is an organic compound that is used as a green yellow pigment and optical brightener. It is classified as a derivative of isoindoline. This yellow green compound is prepared by addition of ammonia to phthalonitrile to give diiminoisoindole, which in turn condenses first with N-methylcyanoacetamide and then with barbituric acid.
Pigment Yellow 13 is an organic compound and an azo compound. It is a widely used yellow pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Yellow 12, wherein the two xylyl groups are replaced by phenyl. It is often depicted as an azo (-N=N-) structure, but according to X-ray crystallography closely related compounds exist as the keto-hydrazide tautomers.

In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.














