
A pigment is a powder used to add color or change visual appearance. Pigments are completely or nearly insoluble and chemically unreactive in water or another medium; in contrast, dyes are colored substances which are soluble or go into solution at some stage in their use. Dyes are often organic compounds whereas pigments are often inorganic. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
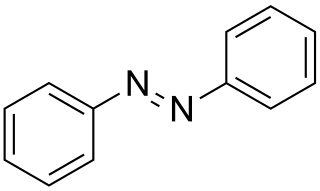
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.

Azo compounds are organic compounds bearing the functional group diazenyl.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
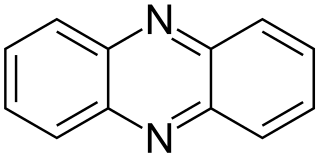
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.
Arylide yellow, also known as Hansa yellow and monoazo yellow, is a family of organic compounds used as pigments. They are primarily used as industrial colorants including plastics, building paints and inks. They are also used in artistic oil paints, acrylics and watercolors. These pigments are usually semi-transparent and range from orange-yellow to yellow-greens. Related organic pigments are the diarylide pigments. Overall, these pigments have partially displaced the toxic cadmium yellow in the marketplace. Painters such as Alexander Calder and Jackson Pollock are known to have employed arylide yellow in their artworks.
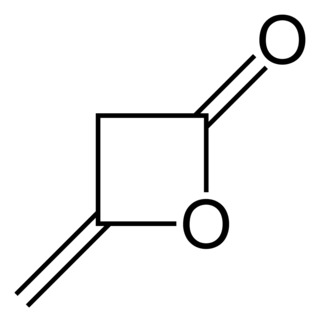
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. Being pigments, these compounds exist as (yellow) powders of low solubility in water. They are similar to the simpler monoazo pigments called arylide yellows.

Pigment Yellow 16 is an organic compound that is classified as a diarylide pigment.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74.
Pigment Yellow 12 is an organic compound and an azo compound. It is a widely used yellow pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Yellow 13, wherein the two phenyl groups are replaced by 2,4-xylyl. According to X-ray crystallography, the molecule is nearly planar and exists as the keto-hydrazide tautomer.

A colorant is any substance that changes the spectral transmittance or reflectance of a material. Synthetic colorants are those created in a laboratory or industrial setting. The production and improvement of colorants was a driver of the early synthetic chemical industry, in fact many of today's largest chemical producers started as dye-works in the late 19th or early 20th centuries, including Bayer AG(1863). Synthetics are extremely attractive for industrial and aesthetic purposes as they have they often achieve higher intensity and color fastness than comparable natural pigments and dyes used since ancient times. Market viable large scale production of dyes occurred nearly simultaneously in the early major producing countries Britain (1857), France (1858), Germany (1858), and Switzerland (1859), and expansion of associated chemical industries followed. The mid-nineteenth century through WWII saw an incredible expansion of the variety and scale of manufacture of synthetic colorants. Synthetic colorants quickly became ubiquitous in everyday life, from clothing to food. This stems from the invention of industrial research and development laboratories in the 1870s, and the new awareness of empirical chemical formulas as targets for synthesis by academic chemists. The dye industry became one of the first instances where directed scientific research lead to new products, and the first where this occurred regularly.
Pigment Orange 13 is an organic compound and an azo compound. It is a commercial orange pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Orange 34, wherein the two phenyl groups are replaced by p-tolyl groups. Its structure has been confirmed by X-ray crystallography.

Pigment Orange 34 is an organic compound and an azo compound. It is a commercial orange pigment, i.e. an insoluble colorant. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Orange 13, wherein the two tolyl groups are replaced by phenyl groups.
Pigment Yellow 97 is widely used as a yellow colorant, and is classified as an arylide yellow. It is distinguished by the presence of a sulfonylaminophenyl group, which renders the material particularly insoluble. It is derived from two fairly complicated precursors. The acetoacetanilide component is made from 2,5-dimethoxy-4-chloroaniline. The azo component is made from a dimethoxyaniline with the sulfonylaminophenyl substituent.he compound is obtained via acetoacetylation of o-tolidine using diketene. The resulting bisacetoacetylated compound is coupled with two equiv of the diazonium salt obtained from 2,4-dichloroaniline. Although it is often depicted as an azo compound, X-ray crystallography shows that the molecule exists as a hydrazone.
Pigment Yellow 14 is an organic compound classified as an azo compound. It is a commercial yellow pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Yellow 13, wherein the two xylyl groups are replaced by an ortho tolyl. It is often depicted as an azo (-N=N-) structure, but according to X-ray crystallography closely related compounds exist as the keto-hydrazide tautomers.












