
The cilium is a short hair-like membrane protrusion from many types of eukaryotic cell. The cilium has the shape of a slender threadlike projection that extends from the surface of the much larger cell body. Eukaryotic flagella found on sperm cells and many protozoans have a similar structure to motile cilia that enables swimming through liquids; they are longer than cilia and have a different undulating motion.

Retinitis pigmentosa (RP) is a member of a group of genetic disorders called inherited retinal dystrophy (IRD) that cause loss of vision. Symptoms include trouble seeing at night and decreasing peripheral vision. As peripheral vision worsens, people may experience "tunnel vision". Complete blindness is uncommon. Onset of symptoms is generally gradual and often begins in childhood.
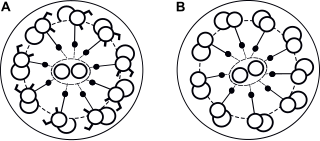
Primary ciliary dyskinesia (PCD) is a rare, autosomal recessive genetic ciliopathy, that causes defects in the action of cilia lining the upper and lower respiratory tract, sinuses, Eustachian tube, middle ear, fallopian tube, and flagella of sperm cells. The alternative name of "immotile ciliary syndrome" is no longer favored as the cilia do have movement, but are merely inefficient or unsynchronized. When accompanied by situs inversus the condition is known as Kartagener syndrome.

In molecular biology, an axoneme, also called an axial filament, is the microtubule-based cytoskeletal structure that forms the core of a cilium or flagellum. Cilia and flagella are found on many cells, organisms, and microorganisms, to provide motility. The axoneme serves as the "skeleton" of these organelles, both giving support to the structure and, in some cases, the ability to bend. Though distinctions of function and length may be made between cilia and flagella, the internal structure of the axoneme is common to both.

X-linked retinitis pigmentosa GTPase regulator is a GTPase-binding protein that in humans is encoded by the RPGR gene. The gene is located on the X-chromosome and is commonly associated with X-linked retinitis pigmentosa (XLRP). In photoreceptor cells, RPGR is localized in the connecting cilium which connects the protein-synthesizing inner segment to the photosensitive outer segment and is involved in the modulation of cargo trafficked between the two segments.
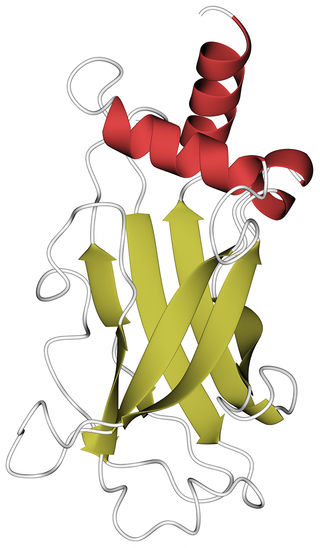
X-linked retinitis pigmentosa GTPase regulator-interacting protein 1 is a protein in the ciliary transition zone that in humans is encoded by the RPGRIP1 gene. RPGRIP1 is a multi-domain protein containing a coiled-coil domain at the N-terminus, two C2 domains and a C-terminal RPGR-interacting domain (RID). Defects in the gene result in the Leber congenital amaurosis (LCA) syndrome and in the eye disease glaucoma.

Dynein axonemal heavy chain 5 is a protein that in humans is encoded by the DNAH5 gene.

Dynein axonemal intermediate chain 1 is a protein that in humans is encoded by the DNAI1 gene.
Conorenal syndrome is a collection of medical conditions that seem to have a common genetic cause.

A ciliopathy is any genetic disorder that affects the cellular cilia or the cilia anchoring structures, the basal bodies, or ciliary function. Primary cilia are important in guiding the process of development, so abnormal ciliary function while an embryo is developing can lead to a set of malformations that can occur regardless of the particular genetic problem. The similarity of the clinical features of these developmental disorders means that they form a recognizable cluster of syndromes, loosely attributed to abnormal ciliary function and hence called ciliopathies. Regardless of the actual genetic cause, it is clustering of a set of characteristic physiological features which define whether a syndrome is a ciliopathy.

Fiona Watt, is a British scientist who is internationally known for her contributions to the field of stem cell biology. In the 1980s, when the field was in its infancy, she highlighted key characteristics of stem cells and their environment that laid the foundation for much present day research.
The Medical Research Council (UK) Human Genetics Unit is situated at the Western General Hospital in Edinburgh. It is one of the largest MRC research establishments, housing over two hundred scientists, support staff, research fellows, PhD students, and visiting workers.

Leucine-rich repeat-containing protein 50 is a protein that in humans is encoded by the LRRC50 gene.

Thioredoxin domain-containing protein 3 (TXNDC3), also known as spermatid-specific thioredoxin-2 (Sptrx-2), is a protein that in humans is encoded by the NME8 gene on chromosome 7.

Dynein axonemal light chain 1, (LC1) is a protein that in humans is encoded by the DNAL1 gene.
A BBSome is a protein complex that operates in primary cilia biogenesis, homeostasis, and intraflagellar transport (IFT). The BBSome recognizes cargo proteins and signaling molecules like G-protein coupled receptors (GPCRs) on the ciliary membrane and helps transport them to and from the primary cilia. Primary cilia are nonmotile microtubule projections that function like antennae and are found in many types of cells. They receive various environmental signals to aid the cell in survival. They can detect photons by concentrating rhodopsin, a light receptor that converts photons into chemical signals, or odorants by concentrating olfactory receptors on the primary cilia surface. Primary cilia are also meaningful in cell development and signaling. They do not contain any way to make proteins within the primary cilia, so the BBSome aids in transporting essential proteins to, from, and within the cilia. Examples of cargo proteins that the BBSome is responsible for ferrying include smoothened, polycystic-1 (PC1), and several G-Protein coupled receptors (GPCRs) like somatostatin receptors (Sstr3), melanin-concentrating hormone receptor 1 (Mchr1), and neuropeptide Y2 receptor.

Ciliogenesis is defined as the building of the cell's antenna or extracellular fluid mediation mechanism. It includes the assembly and disassembly of the cilia during the cell cycle. Cilia are important appendages of cells and are involved in numerous activities such as cell signaling, processing developmental signals, and directing the flow of fluids such as mucus over and around cells. Due to the importance of these cell processes, defects in ciliogenesis can lead to numerous human diseases related to non-functioning cilia known as ciliopathies.

IFT140, Intraflagellar transport 140 homolog, is a protein that in humans is encoded by the IFT140 gene. The gene product forms a core component of IFT-A complex which is indipensible for retrograde intraflagellar transport within the primary cilium.

Robert E. MacLaren FMedSci FRCOphth FRCS FACS VR is a British ophthalmologist who has led pioneering work in the treatment of blindness caused by diseases of the retina. He is Professor of Ophthalmology at the University of Oxford and Honorary Professor of Ophthalmology at the UCL Institute of Ophthalmology. He is a Consultant Ophthalmologist at the Oxford Eye Hospital. He is also an Honorary Consultant Vitreo-retinal Surgeon at the Moorfields Eye Hospital. MacLaren is an NIHR Senior Investigator, or lead researcher, for the speciality of Ophthalmology. In addition, he is a member of the research committee of Euretina: the European Society of Retina specialists, Fellow of Merton College, in Oxford and a Fellow of the Higher Education Academy.















