
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
The Bosch reaction is a catalytic chemical reaction between carbon dioxide (CO2) and hydrogen (H2) that produces elemental carbon (C,graphite), water, and a 10% return of invested heat. CO2 is usually reduced by H2 to carbon in presence of a catalyst (e.g. iron (Fe)) and requires a temperature level of 530–730 °C (986–1,346 °F).
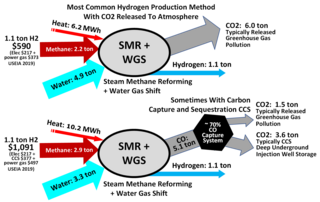
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
Acidogenesis is the second stage in the four stages of anaerobic digestion:

A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ion per unit time make this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
A methane reformer is a device based on steam reforming, autothermal reforming or partial oxidation and is a type of chemical synthesis which can produce pure hydrogen gas from methane using a catalyst. There are multiple types of reformers in development but the most common in industry are autothermal reforming (ATR) and steam methane reforming (SMR). Most methods work by exposing methane to a catalyst at high temperature and pressure.
The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent and compares it to a reference flow of carrier gas. Since most compounds have a thermal conductivity much less than that of the common carrier gases of helium or hydrogen, when an analyte elutes from the column the effluent thermal conductivity is reduced, and a detectable signal is produced.

Methane is a chemical compound with the chemical formula CH4. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
The electrochemical reduction of carbon dioxide, also known as CO2RR, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It represents one potential step in the broad scheme of carbon capture and utilization.
Carbon dioxide reforming is a method of producing synthesis gas from the reaction of carbon dioxide with hydrocarbons such as methane with the aid of noble metal catalysts. Synthesis gas is conventionally produced via the steam reforming reaction or coal gasification. In recent years, increased concerns on the contribution of greenhouse gases to global warming have increased interest in the replacement of steam as reactant with carbon dioxide.
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. Methanizers contain a hydrogenation catalyst to achieve this conversion. Nickel is commonly used as the catalyst and there are alternatives available.
Methane functionalization is the process of converting methane in its gaseous state to another molecule with a functional group, typically methanol or acetic acid, through the use of transition metal catalysts.









