Related Research Articles

In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely divided iron metal as a catalyst:

Pyrolysis is the process of thermal decomposition of materials at elevated temperatures, often in an inert atmosphere without access to oxygen.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
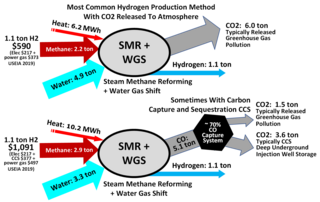
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of ammonia or methanol. The reaction is represented by this equilibrium:

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:
The water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen:

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.

The Boudouard reaction, named after Octave Leopold Boudouard, is the redox reaction of a chemical equilibrium mixture of carbon monoxide and carbon dioxide at a given temperature. It is the disproportionation of carbon monoxide into carbon dioxide and graphite or its reverse:
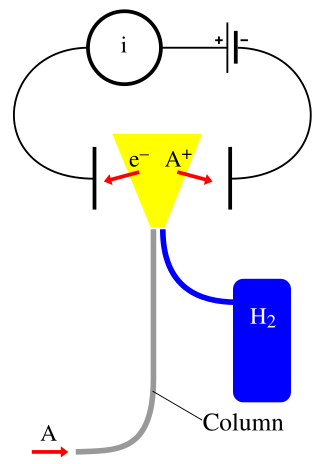
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ions per unit time makes this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments.
Landfill gas monitoring is the process by which gases that are collected or released from landfills are electronically monitored. Landfill gas may be measured as it escapes the landfill or may be measured as it is collected and redirected to a power plant or flare.
A methane reformer is a device based on steam reforming, autothermal reforming or partial oxidation and is a type of chemical synthesis which can produce pure hydrogen gas from methane using a catalyst. There are multiple types of reformers in development but the most common in industry are autothermal reforming (ATR) and steam methane reforming (SMR). Most methods work by exposing methane to a catalyst at high temperature and pressure.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
Methanation is the conversion of carbon monoxide and carbon dioxide (COx) to methane (CH4) through hydrogenation. The methanation reactions of COx were first discovered by Sabatier and Senderens in 1902.
PROX is an acronym for PReferential OXidation, that refers to the preferential oxidation of carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and water-gas shift. An ideal PROX catalyst preferentially oxidizes carbon monoxide (CO) using a heterogeneous catalyst placed upon a ceramic support. Catalysts include metals such as platinum, platinum/iron, platinum/ruthenium, gold nanoparticles as well as novel copper oxide/ceramic conglomerate catalysts.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.
Hydromethanation, [hahy-droh- meth-uh-ney-shuhn] is the process by which methane is produced through the combination of steam, carbonaceous solids and a catalyst in a fluidized bed reactor. The process, developed over the past 60 years by multiple research groups, enables the highly efficient conversion of coal, petroleum coke and biomass into clean, pipeline quality methane.
Carbon dioxide reforming is a method of producing synthesis gas from the reaction of carbon dioxide with hydrocarbons such as methane with the aid of metal catalysts. Synthesis gas is conventionally produced via the steam reforming reaction or coal gasification. In recent years, increased concerns on the contribution of greenhouse gases to global warming have increased interest in the replacement of steam as reactant with carbon dioxide.
A post-column oxidation-reduction reactor is a chemical reactor that performs derivatization to improve the quantitative measurement of organic analytes. It is used in gas chromatography (GC), after the column and before a flame ionization detector (FID), to make the response factor of the detector uniform for all carbon-based species.
References
- ↑ "Methanizer". ARC. Retrieved 2021-03-10.
- ↑ Porter, K.; Volman, D.H. (1962). "Flame Ionization Detection of Carbon Monoxide for Gas Chromatographic Analysis". Anal. Chem. 34 (7): 748–9. doi:10.1021/ac60187a009.
- ↑ Johns, T. and Thompson, B., 16th Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, Mar. 1965.
- ↑ Hightower F.W. and White, A. H., Ind. Eng. Chem. 20 10 (1928)
- ↑ Luong, J.; Yang, Y (2018). "Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices". Anal. Chem. 90 (23): 13815–14094. doi:10.1021/acs.analchem.8b04563. PMID 30411883.
- ↑ Dauenhauer, Paul (January 21, 2015). "Quantitative carbon detector (QCD) for calibration-free, high-resolution characterization of complex mixtures". Lab Chip. 15 (2): 440–7. doi:10.1039/c4lc01180e. PMID 25387003. S2CID 4220408.
- ↑ "Activated Research Company". ARC.