Classical swine fever (CSF) or hog cholera is a highly contagious disease of swine. It has been mentioned as a potential bioweapon.
Circoviridae is a family of DNA viruses. Birds and mammals serve as natural hosts. There are 101 species in this family, assigned to 2 genera. Diseases associated with this family include: PCV-2: postweaning multisystemic wasting syndrome; CAV: chicken infectious anemia.
Mycoplasma hyopneumoniae is a species of bacteria known to cause the disease porcine enzootic pneumonia, a highly contagious and chronic disease affecting pigs. As with other mollicutes, M. hyopneumoniae is small in size (400–1200 nm), has a small genome and lacks a cell wall. It is difficult to grow in laboratories due to its complex nutritional requirements and the high chances of contamination associated with mycoplasma culture. To successfully grow the bacterium, an environment of 5–10% carbon dioxide is required, and the medium should demonstrate an acid colour shift.
Betaarterivirus suid 1, commonly Porcine reproductive and respiratory syndrome virus (PRRSV), is a virus that causes a disease of pigs, called porcine reproductive and respiratory syndrome (PRRS), also known as blue-ear pig disease. This economically important, panzootic disease causes reproductive failure in breeding stock and respiratory tract illness in young pigs.
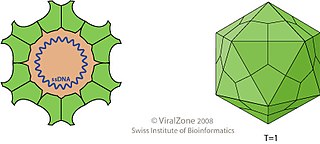
Porcine circovirus (PCV) is a group of four single-stranded DNA viruses that are non-enveloped with an unsegmented circular genome. They are members of the genus Circovirus that can infect pigs. The viral capsid is icosahedral and approximately 17 nm in diameter.
SMEDI is a reproductive disease of swine caused by Porcine parvovirus (PPV) and Porcine enterovirus. The term SMEDI usually indicates Porcine enterovirus, but it also can indicate Porcine parvovirus, which is a more important cause of the syndrome. SMEDI also causes abortion, neonatal death, and decreased male fertility.
Porcine parvovirus (PPV), a virus in the species Ungulate protoparvovirus 1 of genus Protoparvovirus in the virus family Parvoviridae, causes reproductive failure of swine characterized by embryonic and fetal infection and death, usually in the absence of outward maternal clinical signs. The disease develops mainly when seronegative dams are exposed oronasally to the virus anytime during about the first half of gestation, and conceptuses are subsequently infected transplacentally before they become immunocompetent. There is no definitive evidence that infection of swine other than during gestation is of any clinical or economic significance. The virus is ubiquitous among swine throughout the world and is enzootic in most herds that have been tested. Diagnostic surveys have indicated that PPV is the major infectious cause of embryonic and fetal death. In addition to its direct causal role in reproductive failure, PPV can potentiate the effects of porcine circovirus type II (PCV2) infection in the clinical course of postweaning multisystemic wasting syndrome (PMWS).

Psittacine beak and feather disease (PBFD) is a viral disease affecting all Old World and New World parrots. The causative virus—beak and feather disease virus (BFDV)—belongs to the taxonomic genus Circovirus, family Circoviridae. It attacks the feather follicles and the beak and claw matrices of the bird, causing progressive feather, claw and beak malformation and necrosis. In later stages of the disease, feather shaft constriction occurs, hampering development until eventually all feather growth stops. It occurs in an acutely fatal form and a chronic form.

Veterinary virology is the study of viruses in non-human animals. It is an important branch of veterinary medicine.
Actinobacillosis is a zoonotic disease caused by Actinobacillus. It is more commonly associated with animals than with humans.

Foot-and-mouth disease (FMD) or hoof-and-mouth disease (HMD) is an infectious and sometimes fatal viral disease that affects cloven-hoofed animals, including domestic and wild bovids. The virus causes a high fever lasting two to six days, followed by blisters inside the mouth and near the hoof that may rupture and cause lameness.
Iotatorquevirus is a genus of viruses in the family Anelloviridae, in group II in the Baltimore classification. It includes one species: Iotatorquevirus suida1a.
Blue eye disease is caused by La Piedad Michoacán Mexico virus (LPMV), the only member virus of the species Porcine orthorubulavirus in the Paramyxoviridae family. Synonyms for the disease include "Blue Eye Syndrome" and "Porcine Paramyxovirus Blue Eye Disease", and "La Piedad Michoacán Paramyxovirus Infection".
Pacheco's disease is a highly infectious and acute bird disease caused by a species of herpesvirus, Psittacid alphaherpesvirus 1 (PsHV-1). All psittacine species are susceptible to Pacheco's disease, mainly those in zoological collections and aviaries in any geographic regions. Specifically, Pacheco's disease has a high occurrence rate in Amazon parrots, followed by African grey parrots, parrots, macaws, cockatoos and conures. Due to a very high mortality rate within these susceptible species, concerns are brought to companion bird markets and breeders.

Circovirus is a genus of viruses, in the family Circoviridae. Birds and pigs serve as natural hosts, though dogs have been shown to be infected as well. It is a single stranded DNA virus (ssDNA). There are 49 species in this genus. Some members of this genus cause disease: PCV-1 is non pathogenic, while PCV-2 causes postweaning multisystemic wasting syndrome (PMWS).
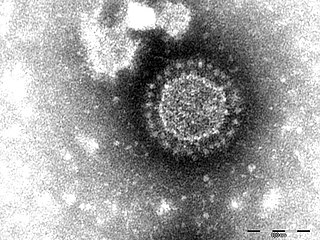
Porcine epidemic diarrhea virus is a coronavirus that infects the cells lining the small intestine of a pig, causing porcine epidemic diarrhoea, a condition of severe diarrhea and dehydration. Older hogs mostly get sick and lose weight after being infected, whereas newborn piglets usually die within five days of contracting the virus. PEDV cannot be transmitted to humans, nor contaminate the human food supply.
Canine circovirus, first isolated in 2012, is a small non-enveloped, icosahedral, single-stranded DNA virus that infects domestic dogs and wild canids exclusively. It is a member of the Circoviridae family and the genus Circovirus. There are currently 11 species of known circoviruses that have been identified to affect a wide variety of birds and mammals. As seen with all extensively studied circoviruses, the diameter ranges between approximately 15 and 25 nanometers. The icosahedral triangulation number is 1, the smallest size a viral capsid can be, in which there are a total of 60 protein subunits that make up the capsid. CaCV is not to be confused with canine coronavirus, another diarrhea-causing agent within the family Coronaviridae, or porcine circoviruses which are a members of the same genus as CaCV but only seen in pigs. CaCV was the first Circovirus to be identified that infects a mammal species other than pigs.
Torque teno sus virus, belonging to the family Anelloviridae, is a group of virus strains that are non-enveloped, with a single-stranded circular DNA genome ranging from 2.6 to 2.8 kb in size. These swine infecting anelloviruses are divided into two genera: Iotatorquevirus and Kappatorquevirus. Torque teno sus virus has been found in pigs worldwide. TTSuVs are mainly transmitted by fecal-oral route. The prevalence of these viruses is relatively high. For now, there is not known disease caused exclusively by TTSuV. There is the possibility that TTSuV may worsen the progression of other diseases and therefore increase the economic losses for pig industry.
Xiang-Jin Meng, also known as X.J. Meng, is a Chinese-born American virologist. He is a university distinguished professor at Virginia Tech. He studies emerging, re-emerging and zoonotic viruses of veterinary and human public health significance. He was elected a member of the National Academy of Sciences in 2016, a Fellow of the National Academy of Inventors in 2014, and a Fellow of the American Academy of Microbiology in 2012.

Alphacoronavirus 1 is a species of coronavirus that infects cats, dogs and pigs. It includes the virus strains feline coronavirus, canine coronavirus, and transmissible gastroenteritis virus. It is an enveloped, positive-strand RNA virus which is able to enter its host cell by binding to the APN receptor.





