
Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
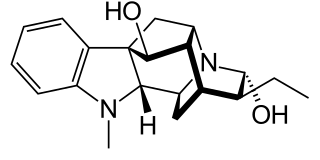
Ajmaline is an alkaloid that is classified as a 1-A antiarrhythmic agent. It is often used to induce arrhythmic contraction in patients suspected of having Brugada syndrome. Individuals suffering from Brugada syndrome will be more susceptible to the arrhythmogenic effects of the drug, and this can be observed on an electrocardiogram as an ST elevation.

Plakohypaphorines are halogenated indolic non-proteinogenic amino acids named for their similarity to hypaphorine (N,N,N-trimethyltryptophan). First reported in the Caribbean sponge Plakortis simplex in 2003, plakohypaphorines A-C were the first iodine-containing indoles to be discovered in nature. Plakohypaphorines D-F, also found in P. simplex, were reported in 2004 by a group including the researchers who discovered the original plakohypaphorines.

7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom. It is often referred to as ‘7-OH’. It was first described in 1994 and is a natural product derived from the mitragynine present in the Kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine.
In enzymology, a secologanin synthase (EC 1.14.19.62, was wrongly classified as EC 1.3.3.9 in the past) is an enzyme that catalyzes the chemical reaction

Yuremamine is a phytoindole alkaloid which was isolated from the bark of Mimosa tenuiflora in 2005, and erroneously assigned a pyrrolo[1,2-a]indole structure that was thought to represent a new class of indole alkaloids. However, in 2015, the bioinspired total synthesis of yuremamine revealed its structure to be a flavonoid derivative. It was also noted in the original isolation of yuremamine that the alkaloid occurs naturally as a purple solid, but total synthesis revealed that yuremamine as a free base is colorless, and the formation of a trifluoroacetate salt during HPLC purification is what led to the purple appearance.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to both yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.

Pericine is one of a number of indole alkaloids found in the tree Picralima nitida, commonly known as akuamma. As with some other alkaloids from this plant such as akuammine, pericine has been shown to bind to mu opioid receptors in vitro, and has an IC50 of 0.6 μmol, within the range of a weak analgesic. It may also have convulsant effects.
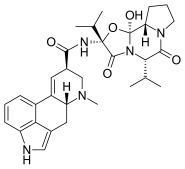
Ergocornine is a crystalline ergopeptine and one of the ergot alkaloids separated from ergotoxine. It is also a dopamine receptor agonist. It was discovered by Albert Hofmann, the Swiss chemist who created LSD.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

N-Methylserotonin is a tryptamine alkaloid. Chemically, it is a derivative of serotonin in which a methyl group resides at its alkyl amine. It is also called Nω-methylserotonin (Nω-methyl-5-hydroxytryptamine) to distinguish it from tryptamine-derived compounds in which a methyl group is bonded to the nitrogen atom of the indole group.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Affinine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally it can be considered a member of the vobasine alkaloid family and may be synthesized from tryptophan. Limited pharmacological testing has indicated that it may be an effective inhibitor of both acetylcholinesterase and butyrylcholinesterase.
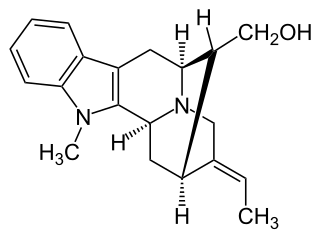
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
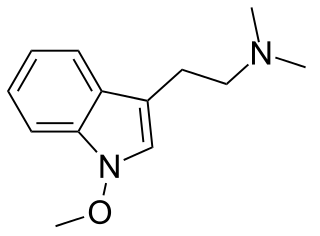
Lespedamine is an indole alkaloid and substituted tryptamine present in the plant Lespedeza bicolor. The alkaloid bears a close structural resemblance to the psychedelic alkaloid dimethyltryptamine and was speculated to have psychoactivity by Alexander Shulgin. No reports on lespedamine's biological activity have been published.

Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine. The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways such as in Aspidosperma species. Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are:

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.



















