
Fire blight, also written fireblight, is a contagious disease affecting apples, pears, and some other members of the family Rosaceae. It is a serious concern to apple and pear producers. Under optimal conditions, it can destroy an entire orchard in a single growing season.

Plum pox, also known as sharka, is the most devastating viral disease of stone fruit from the genus Prunus. The disease is caused by the plum pox virus (PPV), and the different strains may infect a variety of stone fruit species including peaches, apricots, plums, nectarine, almonds, and sweet and tart cherries. Wild and ornamental species of Prunus may also become infected by some strains of the virus.

Lettuce mosaic virus (LMV) is a typical potyvirus, which causes one of the major virus diseases of lettuce crops worldwide.

Papaya ringspot virus (PRSV) is a pathogenic plant virus in the genus Potyvirus and the virus family Potyviridae which primarily infects the papaya tree.
Xanthomonas arboricola is a species of bacteria. This phytopathogenic bacterium can cause disease in trees like Prunus, hazelnut and walnut.

Apple mosaic virus (ApMV) is a plant pathogenic virus of the family Bromoviridae. It is named after its symptoms that were first present on apples. ApMV is a positive sense RNA based virus. The disease itself has several synonyms including Mild Apple Mosaic Virus, Hop Virus, Rose Mosaic Virus, and European Plum Line Patten Virus. It causes a severe yield reduction and decreased life-expectancy of fruit trees.
Barley stripe mosaic virus (BSMV), of genus Hordevirus, is an RNA viral plant pathogen whose main hosts are barley and wheat. The common symptoms for BSMV are yellow streaks or spots, mosaic, leaves and stunted growth. It is spread primarily through infected seed and can be spread through mechanical transfer of an infected and uninfected host. Plants infected with BSMV are more symptomatic in warmer temperatures. Resistant hosts and sterilization of equipment are the best ways to control the spread of the pathogen. BSMV has been known to reduce the yields of barley by up to 25%, but is not a major problem because of resistant varieties of barley.

Beet curly top virus (BCTV) is a pathogenic plant virus of the family Geminiviridae, containing a single-stranded DNA. The family Geminiviridae consists of nine genera based on their host range, virus genome structure, and type of insect vector. BCTV is a Curtovirus affecting hundreds of plants. The only known vector is the beet leafhopper, which is native to the Western United States.
Cherry mottle leaf virus (CMLV) is a plant pathogenic virus causing leaf rot. It is closely related to the peach mosaic virus.

Impatiens necrotic spot orthotospovirus(INSV) is a plant pathogenic virus of the order Bunyavirales. It was originally believed to be another strain of Tomato spotted wilt virus, but genetic investigations revealed them to be separate viruses. It is a negative-strand RNA virus which has a tripartite genome. It is largely spread by the insect vector of the western flower thrips. The virus infects more than 648 species of plants including important horticultural and agricultural species such as fuchsia, tomato, orchids, and lettuce (especially romaine). As the name implies, the main symptom on plants is necrotic spots that appear on the leaves. The INSV virus infects by injecting the RNA the virus contains into the cell which then starts using the cell resources to transcribe what the virus RNA states. Viral infection can often result in the death of the plant. The disease is mainly controlled by the elimination of the western flower thrip vector and by destroying any infected plant material.
Prune dwarf virus (PDV) is an economically important plant pathogenic virus affecting Prunus species globally. PDV is found worldwide due to easy transmission through seed, pollen, and vegetative propagation. The virus is in the family Bromoviridae an important family of plant RNA viruses containing six genera, including Alfamovirus, Ilarvirus, Bromovirus, Amularvirus, Oleavirus, and Cucumovirus. PDV belongs to the genera Ilarvirus. It can cause dwarfism of leaves on certain prune and plum plants. It will also cause yellows in sour cherry, especially when present with prunus necrotic ringspot virus. There are no known transmission vectors, though the pollen of infected cherry trees has been found to infect other cherry trees a small percent of the time.
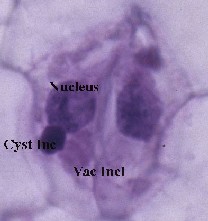
Tobacco ringspot virus (TRSV) is a plant pathogenic virus in the plant virus family Secoviridae. It is the type species of the genus Nepovirus. Nepoviruses are transmitted between plants by nematodes, thrips, mites, grasshoppers, and flea beetles. TRSV is also easily transmitted by sap inoculation and transmission in seeds has been reported. In recent cases it has also been shown to appear in bees, but no transmission to plants from bees has been noted.

Tobacco streak virus (TSV) is a plant pathogenic virus of the family Bromoviridae, in the genus Ilarvirus. It has a wide host range, with at least 200 susceptible species. TSV is generally more problematic in the tropics or warmer climates. TSV does not generally lead to epidemics, with the exception of sunflowers in India and Australia, and peanuts in India.

Soybean mosaic virus (SMV) is a member of the plant virus genus Potyvirus. It infects mainly plants belonging to the family Fabaceae but has also been found infecting other economically important crops. SMV is the cause of soybean mosaic disease that occurs in all the soybean production areas of the world. Soybean is one of the most important sources of edible oil and proteins and pathogenic infections are responsible for annual yield losses of about $4 billion in the United States. Among these pathogens, SMV is the most important and prevalent viral pathogen in soybean production worldwide. It causes yield reductions of about 8% to 35%, but losses as high as 94% have been reported.

Orthotospovirus is a genus of negative-strand RNA viruses, in the family Tospoviridae of the order Bunyavirales, which infects plants. Tospoviruses take their name from the species Tomato spotted wilt orthotospovirus (TSWV) which was discovered in Australia in 1919. TSWV remained the only known member of the family until the early 1990s when genetic characterisation of plant viruses became more common. There are now at least twenty species in the genus with more being discovered on a regular basis. Member viruses infect over eight hundred plant species from 82 different families.
Black rot, caused by the bacterium Xanthomonas campestris pv. campestris (Xcc), is considered the most important and most destructive disease of crucifers, infecting all cultivated varieties of brassicas worldwide. This disease was first described by botanist and entomologist Harrison Garman in Lexington, Kentucky, US in 1889. Since then, it has been found in nearly every country in which vegetable brassicas are commercially cultivated.

Beet vascular necrosis and rot is a soft rot disease caused by the bacterium Pectobacterium carotovorum subsp. betavasculorum, which has also been known as Pectobacterium betavasculorum and Erwinia carotovora subsp. betavasculorum. It was classified in the genus Erwinia until genetic evidence suggested that it belongs to its own group; however, the name Erwinia is still in use. As such, the disease is sometimes called Erwinia rot today. It is a very destructive disease that has been reported across the United States as well as in Egypt. Symptoms include wilting and black streaks on the leaves and petioles. It is usually not fatal to the plant, but in severe cases the beets will become hollowed and unmarketable. The bacteria is a generalist species which rots beets and other plants by secreting digestive enzymes that break down the cell wall and parenchyma tissues. The bacteria thrive in warm and wet conditions, but cannot survive long in fallow soil. However, it is able to persist for long periods of time in the rhizosphere of weeds and non-host crops. While it is difficult to eradicate, there are cultural practices that can be used to control the spread of the disease, such as avoiding injury to the plants and reducing or eliminating application of nitrogen fertilizer.
Little cherry disease or LChD, sometimes referred to as little cherry, K & S little cherry or sour cherry decline, is a viral infectious disease that affects cherry trees, most notably sweet cherries and sour cherries . Little cherry disease should not be confused with cherry buckskin disease, which is caused by Phytoplasma. Note that both diseases are among the diseases referred to as cherry decline.

Melon necrotic spot virus (MNSV) is a virus that belongs to the genus Gammacarmovirus of the family Tombusviridae. It has been observed in several countries of the Americas, Africa, Asia, and Europe. It is considered to be an endemic virus in greenhouses and field productions of Cucurbitaceae crops, including melon, cucumber, and watermelon. MNSV is mainly spread through infected soil, seedlings, insects, and by the root-inhabiting fungus vector Olpidium bornovanus. Symptoms vary between Curbitaceae crops, but generally consist of chlorosis, brown necrotic lesions, leaf wilt, fruit decay, and plant death. Management of the disease consists of preventing infection by rotating fields and crops, steam sterilization, and disposal of infected plants. Also, treated seeds with heat or chemicals are efficient in preventing infection. MNSV is important in melon plants as it causes vast economical damage worldwide reducing significant yields.
Soybean vein necrosis orthotospovirus is a plant pathogenic virus of soybeans. SVNV is a relatively new virus, which was discovered in Tennessee in 2008 and has recently been found in many US states from the Southeast and East coast to some western states including CA. This pathogen initially causes intraveinal chlorosis (yellowing) in leaves. This chlorosis then spreads throughout the leaf and eventually these chlorotic areas can become necrotic. It is a member of the order Bunyavirales, family Tospoviridae and genus Orthotospovirus, which is the only genus within this virus family that infects plants. Like other members of Bunyavirales, this virus is enveloped and has a negative sense single-stranded RNA (−ssRNA) genome composed of three genomic segments. It encodes proteins on the M and S segments in an ambisense manner.














