Related Research Articles

Sandstone is a clastic sedimentary rock composed mainly of sand-sized silicate grains. Sandstones comprise about 20–25% of all sedimentary rocks.

Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2Si2O5(OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite. Shale is characterized by its tendency to split into thin layers (laminae) less than one centimeter in thickness. This property is called fissility. Shale is the most common sedimentary rock.

Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles to settle in place. The particles that form a sedimentary rock are called sediment, and may be composed of geological detritus (minerals) or biological detritus. The geological detritus originated from weathering and erosion of existing rocks, or from the solidification of molten lava blobs erupted by volcanoes. The geological detritus is transported to the place of deposition by water, wind, ice or mass movement, which are called agents of denudation. Biological detritus was formed by bodies and parts of dead aquatic organisms, as well as their fecal mass, suspended in water and slowly piling up on the floor of water bodies. Sedimentation may also occur as dissolved minerals precipitate from water solution.
Vitrinite is one of the primary components of coals and most sedimentary kerogens. Vitrinite is a type of maceral, where "macerals" are organic components of coal analogous to the "minerals" of rocks. Vitrinite has a shiny appearance resembling glass (vitreous). It is derived from the cell-wall material or woody tissue of the plants from which coal was formed. Chemically, it is composed of polymers, cellulose and lignin.

Metamorphism is the transformation of existing rock to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of 150 to 200 °C, and often also at elevated pressure or in the presence of chemically active fluids, but the rock remains mostly solid during the transformation. Metamorphism is distinct from weathering or diagenesis, which are changes that take place at or just beneath Earth's surface.

Sekaninaite ((Fe+2,Mg)2Al4Si5O18) is a silicate mineral, the iron-rich analogue of cordierite.

Migmatite is a composite rock found in medium and high-grade metamorphic environments, commonly within Precambrian cratonic blocks. It consists of two or more constituents often layered repetitively: one layer is an older metamorphic rock that was reconstituted subsequently by partial melting ("paleosome"), while the alternate layer has a pegmatitic, aplitic, granitic or generally plutonic appearance ("neosome"). Commonly, migmatites occur below deformed metamorphic rocks that represent the base of eroded mountain chains.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Granulites are a class of high-grade metamorphic rocks of the granulite facies that have experienced high-temperature and moderate-pressure metamorphism. They are medium to coarse–grained and mainly composed of feldspars sometimes associated with quartz and anhydrous ferromagnesian minerals, with granoblastic texture and gneissose to massive structure. They are of particular interest to geologists because many granulites represent samples of the deep continental crust. Some granulites experienced decompression from deep in the Earth to shallower crustal levels at high temperature; others cooled while remaining at depth in the Earth.

Lechatelierite is silica glass, amorphous SiO2, non-crystalline mineraloid.
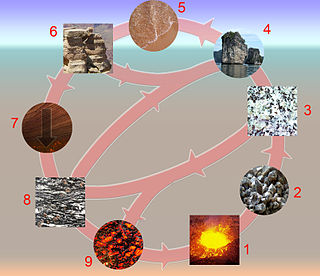
The rock cycle is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium conditions. For example, an igneous rock such as basalt may break down and dissolve when exposed to the atmosphere, or melt as it is subducted under a continent. Due to the driving forces of the rock cycle, plate tectonics and the water cycle, rocks do not remain in equilibrium and change as they encounter new environments. The rock cycle explains how the three rock types are related to each other, and how processes change from one type to another over time. This cyclical aspect makes rock change a geologic cycle and, on planets containing life, a biogeochemical cycle.
Restite is the residual material left at the site of melting during the in place production of granite through intense metamorphism.
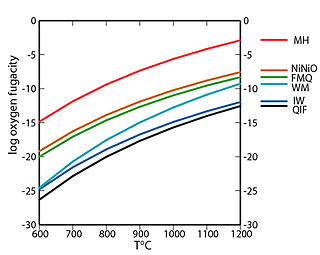
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
This glossary of geology is a list of definitions of terms and concepts relevant to geology, its sub-disciplines, and related fields. For other terms related to the Earth sciences, see Glossary of geography terms.
Sulfate crust is a zone observed in the axial (central) parts of burning coal dumps and related sites. It is a zone built mainly by anhydrous sulfate minerals, such as godovikovite and millosevichite. The outer zone can easily be hydrated giving rise to minerals like tschermigite and alunogen. The zone forms due to interaction with hot (even around 600 °C) coal-derived gases (mainly NH3 and SO3) with the "sterile" material (i.e. shales and other rocks serving as the source of Al3+, Fe3+, Ca2+ and other cations) in case of the lack of vents for the gases to escape into the atmosphere.
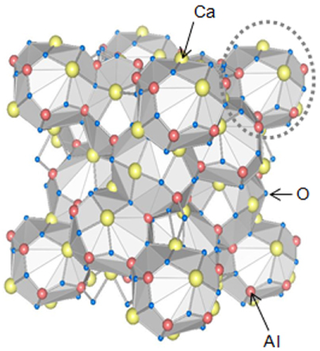
Chlormayenite (after Mayen, Germany), Ca12Al14O32[☐4Cl2], is a rare calcium aluminium oxide mineral of cubic symmetry.
The Hatrurim Formation or Mottled Zone is a geologic formation with outcrops all around the Dead Sea Basin: in the Negev Desert in Israel, in the Judaean Desert on the West Bank, and in western Jordan. It includes late Cretaceous to Eocene aged impure limestone along with coal bearing chalk and marl. The rocks have been subjected to pyrometamorphism resulting from combustion of contained or underlying coal or hydrocarbon deposits. The formation is named for exposures in the Hatrurim Basin which lies west of the Dead Sea.

Esseneite is a relative rare mineral of the pyroxene group, with formula CaFeAlSiO6. It is the ferric-iron-dominant member. Esseneite is an iron-analogue of other pyroxene-group members, davisite, grossmanite, and kushiroite. It is a metamorphic mineral forming in pyrometamorphic rocks called paralavas, which are formed due to fusing on sedimentary rocks usually in result of coal fires. Esseneite is found in both natural and anthropogenic coal-fire sites.
Nabimusaite is a very rare mineral with formula KCa12(SiO4)4(SO4)2O2F. Its structure, as in case of similar aradite and zadovite, is a derivative of the one of hatrurite. Nabimusaite gives its name to the nabimusaite group. The mineral was found in a pyrometamorphic rock of the Hatrurim Formation, a site known for the natural pyrometamorphism. It is interpreted to have formed due to interaction of a precursor assemblage with sulfate-rich melt. Nabimusaite is potassium- and fluorine-analogue of dargaite.
References
- ↑ Grapes, R.H., 2006. Pyrometamorphism. Springer Verlag, Berlin
- ↑ Sokol, E.V., Maksimova, N.V., Nigmatulina, E.N., Sharygin, V.V., and Kalugin, V.M., 2005. Combustion metamorphism. Publishing House of the Siberian Branch of the Russian Academy of Sciences, Novosibirsk (in Russian, with parts in English)
- ↑ Simmons, W.B., Cosca, M.A., Essene, E.J., and Coates, D.A., 1989. Pyrometamorphic rocks associated with naturally burned coal beds, Powder River Basin, Wyoming. American Mineralogist 74, 85-100
- ↑ Grapes, R., Zhang, K., and Peng, Z., 2009. Paralava and clinker products of coal combustion, Yellow River, Shanxi Province, China. Lithos 113(3-4), 831-843
- ↑ Sharygin, V.V., Sokol, E.V., and Belakovskii, D.I., 2009. Fayalite-sekaninaite paralava from the Ravat coal fire (central Tajikistan). Russian Geology and Geophysics 50(8), 703-721
- ↑ Mindat, http://www.mindat.org