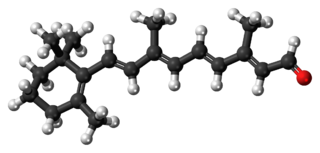
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
The visual cycle is a process in the retina that replenishes the molecule retinal for its use in vision. Retinal is the chromophore of most visual opsins, meaning it captures the photons to begin the phototransduction cascade. When the photon is absorbed, the 11-cis retinal photoisomerizes into all-trans retinal as it is ejected from the opsin protein. Each molecule of retinal must travel from the photoreceptor cell to the RPE and back in order to be refreshed and combined with another opsin. This closed enzymatic pathway of 11-cis retinal is sometimes called Wald's visual cycle after George Wald (1906–1997), who received the Nobel Prize in 1967 for his work towards its discovery.
In enzymology, a retinol dehydrogenase (RDH) (EC 1.1.1.105) is an enzyme that catalyzes the chemical reaction
In enzymology, an all-trans-retinol 13,14-reductase is an enzyme, encoded by the RETSAT gene, that catalyzes the chemical reaction

RPE-retinal G protein-coupled receptor also known as RGR-opsin is a protein that in humans is encoded by the RGR gene. RGR-opsin is a member of the rhodopsin-like receptor subfamily of GPCR. Like other opsins which bind retinaldehyde, it contains a conserved lysine residue in the seventh transmembrane domain. RGR-opsin comes in different isoforms produced by alternative splicing.

S-arrestin is a protein that in humans is encoded by the SAG gene.

11-cis retinol dehydrogenase is an enzyme that in humans is encoded by the RDH5 gene.

Arrestin-C, also known as retinal cone arrestin-3, is a protein that in humans is encoded by the ARR3 gene.

Alcohol dehydrogenase 4 is an enzyme that in humans is encoded by the ADH4 gene.

Guanine nucleotide-binding protein G(T) subunit gamma-T1 is a protein that in humans is encoded by the GNGT1 gene. Either GNGT1 or GNGT2 is the gamma subunit (Gγ) of the Gβγ part of transducin, a heterotrimeric G-protein naturally expressed in vertebrate retina rod and cone cells. GNGT1 only occurs in rod cells, and GNGT2 only occurs in cone cells, with a different alpha (Gα) subunit.

Retinol dehydrogenase 11 is an enzyme that in humans is encoded by the RDH11 gene.

Retinol dehydrogenase 12 is an enzyme that in humans is encoded by the RDH12 gene.

Retinol dehydrogenase 14 is an enzyme that in humans is encoded by the RDH14 gene.

Short-chain dehydrogenase/reductase 3 is an enzyme that in humans is encoded by the DHRS3 gene.

Dehydrogenase/reductase SDR family member 9 is an enzyme that in humans is encoded by the DHRS9 gene.

Hydroxysteroid 17-beta dehydrogenase 6 is an enzyme that in humans is encoded by the HSD17B6 gene.

CYP27C1 is a protein that in humans is encoded by the CYP27C1 gene. The Enzyme Commission number (EC) for this protein is EC 1.14.19.53. The full accepted name is all-trans-retinol 3,4-desaturase and the EC number 1 classifies CYP27C1 as a oxidoreductase that acts on paired donor by reducing oxygen. It is also identifiable by the UniProt code Q4G0S4.

Lecithin retinol acyltransferase is an enzyme that in humans is encoded by the LRAT gene.

Retinol dehydrogenase 13 (all-trans/9-cis) is a protein that in humans is encoded by the RDH13 gene. This gene encodes a mitochondrial short-chain dehydrogenase/reductase, which catalyzes the reduction and oxidation of retinoids. The encoded enzyme may function in retinoic acid production and may also protect the mitochondria against oxidative stress. Alternatively spliced transcript variants have been described.

Emixustat is a small molecule notable for its establishment of a new class of compounds known as visual cycle modulators (VCMs). Formulated as the hydrochloride salt, emixustat hydrochloride, it is the first synthetic medicinal compound shown to affect retinal disease processes when taken by mouth. Emixustat was invented by the British-American chemist, Ian L. Scott, and is currently in Phase 3 trials for dry, age-related macular degeneration (AMD).
















